|
Table of Content - Volume 21 Issue 3 - March 2022
Original Research Article
Original Research Article
A Randomized prospective comparative Study of 2-chloroprocaine 1% against hyperbaric Bupivacaine 0.5% for a subarachnoid block in parturient women scheduled for an elective caesarean delivery at a tertiary care hospital
Sajidhusain B N1*, Faizan A B2
1Department of Anaesthesiology, Belgaum Institute of Medical Sciences, B.R Ambedkar Road, Belgaum, Karnataka, India-590001. 2Ph.D. Research Scholar, Department of Pharmacology and Toxicology, KLE College Of Pharmacy, Belagavi. A Constitute Unit of KLE Academy Higher Education and Research, Belagavi-590010, INDIA. Email: sajidnadaf@gmail.com
Abstract Background: For patients receiving elective LSCS, spinal anaesthesia has proven to be a safe way to assure appropriate analgesia. A large variety of intrathecal products, as well as a broad range of adjuvants, have been studied over many years. In this study, we compared subarachnoid block with 1% 2-chloroprocaine versus hyperbaric 0.5% Bupivacaine in pregnant women scheduled for an elective caesarean section. Aim: The researcher is able to see if a subarachnoid block with 1% 2-chloroprocaine versus 0.5% hyperbaric bupivacaine was a safe technique to ensure optimal analgesia in pregnant women scheduled for an elective caesarean section anaesthetic. Material and Methods: The present study was a prospective, comparative, single-centre study in parturient females aged 18 to 40 years old with an ASA status of ≤ 2 who were scheduled for elective LSCS under Spinal anaesthesia. N=80 patients were allocated into two groups randomly (by chit method): Group B (received 2ml of 0.5% hyperbaric Bupivacaine) and Group C (received 2.5 ml of preservative-free 1% 2-Chloroprocaine). Results: In this study, n = 80 patients were divided into two groups: group B (n = 40) and group C (n = 40). Age, weight, height, ASA grade (I/II), and surgery length were comparable in both groups, with no statistically significant differences. When compared to the Bupivacaine group, the Chloroprocaine group had an earlier onset of sensory block (2.01± 1.09 min vs. 3.36± 1.2 min), an onset of motor block (3.71± 1.25 min vs. 5.13± 1.47 min), and less time to achieve sensory blockade at its maximum level (3.01± 0.92 min vs. 5.08± 0.75 min), and the difference was statistically significant (p<0.001). Conclusion: In elective LSCS, the use of 1% 2 Chloroprocaine is a safe and effective alternative to Bupivacaine, with a faster onset, predictable sensory block height, appropriate motor block duration, and duration of analgesia. Keywords: 1% 2-chloroprocaine, 0.5% hyperbaric bupivacaine, spinal anaesthesia and elective caesarean section.
INTRODUCTION In underdeveloped countries, LSCS is one of the most commonly performed obstetric operations in the parturient population. LSCS improves maternal and foetal outcomes as well as lowers the risks of spontaneous labour and vaginal delivery. Due to its easy capacity to deliver enough surgical anaesthetic, ease and simplicity of technique, quicker onset of action, and safety, Spinal anaesthesia has proven to be a safe technique and assures adequate analgesia for patients undergoing elective LSCS.1 When compared to general anaesthesia, regional anaesthesia is a safer option for both the mother and the newborn during a caesarean section. Hence Subarachnoid block (SAB) is the preferred regional anaesthetic approach for elective caesarean section and its advantages are many such as ease of use, low cost, early-onset, capacity to deliver appropriate surgical anaesthesia, hence there is reduced new-born depression, fewer problems, and low failure rate. A wide range of intrathecal products, as well as a wide range of adjuvants, have been studied over many years. The optimal local anaesthetic should have a fast start of action, a faster offset of motor blockage with predictable duration, appropriate postoperative pain control, low neurotoxicity potential, and minimal systemic adverse effects.2 2-chloroprocaine (2-CP) is an amino-ester local anaesthetic (LA) available as preservative-free LA. It has a rapid onset, effective sensory and motor block, a short recovery time, and few side effects3. Intrathecal LA with adjuvant drugs increases the quality and duration of the spinal blockade and extends postoperative analgesia. By using an adjuvant, it is possible to lower the amount of LA and, consequently, the occurrence of negative effects. Similar, to the parturient receiving elective LSCS, bupivacaine is the most commonly used local anaesthetic for spinal anaesthesia. Bupivacaine is a long-acting amide local anaesthetic that provides effective pain relief without having a significant effect on motor fibres4, 5. Its duration of action is 1 and a half to 2 hours. Because of its rapid onset and brief duration of action, predictable block height, and time to complete regression, the antioxidant and preservative-free version of 1% 2chloroprocaine is now again available for use in subarachnoid blocks6. The current study compared subarachnoid block with 1% 2-chloroprocaine versus 0.5 % hyperbaric bupivacaine in pregnant women scheduled for an elective caesarean section at a tertiary hospital.
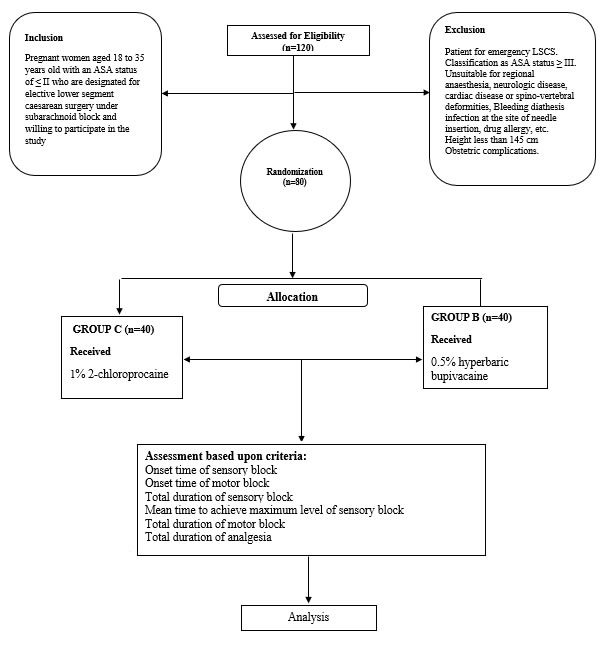
METHODS The study was a randomised, hospital-based, comparative study and was conducted at the departments of anaesthesiology in a tertiary care hospital for a period of two year (July 2018 to June 2019). With the signed consent of subjects undergoing operative procedures and receiving both, compared subarachnoid block with 1% 2-chloroprocaine versus 0.5 % hyperbaric bupivacaine were considered for the study. A total of n=80 subjects were enrolled based on the inclusion criteria, making the final tally n=80 subjects with a 100% completion rate. The foregoing were the criteria seeking inclusion: Pregnant women aged 18 to 35 years old with an ASA status of ≤2 who are designated to undergo elective LSCS under the subarachnoid block and willing to participate in the study. The following were the criteria for exclusion: Patient for emergency LSCS. Classification as ASA status ≥ III. Unsuitable for regional anaesthesia, neurologic disease, spinal deformities, cardiac diseases, infection at the needle insertion site, drug allergy, etc. Medical disorders - Pre-eclampsia, Gestational diabetes mellitus, thyroid disorders, anaemia. Height < 145 cm, Body mass index (BMI) ≥ 30 kg/m2 Obstetric complications - Multiple gestations, Polyhydramnios, oligohydramnios, placenta previa, bleeding diathesis, premature membrane rupture; premature delivery, foetal anomaly; intrauterine growth restriction. Patients were assigned to either the control or interventional group through a concealed allocation method. The control group (subject and the guardian) received normal medical care, while in the experimental/intervention group, the guardians were provided with verbal and written information about anaesthesia surgery, its benefits, harms, and on-going needs. In India, the current status of scientific progression in the field, etc., along with physician consultation and their counselling, Patient counselling on generalised health, as all patients underwent history taking, present symptoms, and past medical/surgical history, were evaluated for routine investigations, and posted for surgery after anaesthetic fitness. Patients were given a signed informed consent form after the procedure was explained to them in their native language. A thorough medical history was taken, as well as a thorough general physical and systemic examination, which included an airway assessment and a spine examination. The necessary laboratory tests (CBC, BT, CT, LFT, and RFT) were performed when requested. Preoperatively, patients were kept nil per oral for 6 hours. Further, n=80 patients were randomly divided (by chit method) into: Group B - received 2ml of 0.5% hyperbaric bupivacaine Group C - received 2.5 ml of preservative-free 1% 2-chloroprocaine. When the patient arrived in the operating room, an intravenous line was set up and 500 mL of Ringer lactate solution was preloaded during a 20-to-30 minute period. The patients' basic vital values were recorded. Aseptic precautions were taken, a subarachnoid block was performed, and the medication was delivered intrathecally according to the group allocation. Before the procedure, the patient's pulse, NIBP, SpO2, and respiratory rate were monitored every 5 minutes until the patient was moved out of the recovery room. The period between the time of intrathecal injection and the development of loss of sensation to pinprick was recorded as the time of origin of sensory block, while the motor block was evaluated using the modified Bromage scale. During surgery, adverse effects such as nausea, vomiting, hypotension, bradycardia, respiratory distress, and shivering patients were carefully monitored, if any and treated as needed. Post-surgery, any complications such as headaches, backaches, nausea, vomiting, urinary retention, or symptoms or signs of TNS were documented and treated appropriately. (TNS was defined as pain/dysaesthesia originating in the buttocks and radiating to the thigh and legs, occurring within 24 hours of spinal administration).
Schematic Diagram Flow chart
STATISTICAL ANALYSIS The data was collected offline and then sorted and organised into clinical and demographical data sections for both the control and experimental groups using Microsoft Excel. The data was analysed using SPSS v23.0. For categorical data, frequency, percentage, mean, and standard deviations (SD) were calculated, whilst ratios and proportions were also calculated. The difference in proportions across qualitative variables was examined using either the chi-square test or the Fisher exact test, as appropriate. A P-value less than 0.5 was considered statistically significant. Quantitative data was shown in ranges and means with standard deviations, while qualitative variables were shown in frequencies and percentages. The ASA grade was shown in numbers and percentages. Age, height, weight, and the onset and duration of sensory and motor blocks were represented using means and standard deviations. The maximum level of sensory blockade, the motor blockade duration, the duration of analgesia, and complications were displayed as figures and percentages.
RESULTS In the present study, N=80 subjects were divided into Group C (n=40) and Group B (n=40) by random allocation (Chit method). Age, height and weight are seen as comparable in both groups. ASA grading in Group C was shown to be exhibited with n=30 with Grade ASA-I and n=10 with Grade ASA-II, similarly with that of Group B shown to have n=31 with Grade ASA- I and n=9 in Grade ASA-II respectively. The duration of surgery in minutes was seen, as in Group C with 37.72 ± 11.82 and in group B with 36.23 ± 12.25. The difference was not statistically significant as shown in Table 1.
Table 1: General characteristics
P<0.05
The present study also assessed the Chloroprocaine group had early onset of sensory block (2.01 ± 1.09 min vs 3.36 ± 1.2 min), the onset of motor block (3.71 ± 1.25 min vs 5.13 ± 1.47 min) and less time to achieve the maximum level of sensory block (3.01 ± 0.92 min vs 5.08 ± 0.75 min) as compared to bupivacaine group and the difference was seen clinically and statistically significant <0.001 with p<0.05. While in the Bupivacaine group longer duration of sensory block (76.74 ± 11.94 min vs 168.60 ± 12.41 min), longer duration of motor block (98.27 ± 28.3 min vs 164.82 ± 23.47 min) and longer duration of analgesia (109.32 ± 25.8 min vs 189.28 ± 39.76 min) as compared to chloroprocaine group and the difference was seen clinically and statistically significant p <0.001 with p<0.05 as shown in Table 2. Table 2: Anaesthesia characteristics
P<0.05
The common side effects such as Hypotension was seen as a highest in Group B with n=11 (27.5%) and Group C with n=8 (20%), followed by bradycardia as n=2 (5%) in Group C and n=8 (20%) in Group B, as vomiting with n=3 (7.5%) with n=1 (2.5%) and least with nausea as n=2 (5%) and n=1 (2.5%) respectively. As it was noted in both groups and difference was statistically not significant No transient neurological symptoms were noted till discharge. No morbidity or mortality was noted in the present study. No patient required conversion into general anaesthesia as shown in Table 3.
Table 3: Side Effects
P<0.05
DISCUSSION The growing presence of managed care in the healthcare sector creates greater incentives to provide high-quality, cost-effective medical treatment. The main outcomes of our study were that using 1% 2-Chloroprocaine for the subarachnoid block instead of 0.5% hyperbaric Bupivacaine enhanced the sensory block and duration of postoperative analgesia. When compared to epidural anaesthesia, spinal blocks offer the added benefit of being less expensive. The cost difference was attributable to epidurals' greater complication rate and significantly longer overall operating room durations due to epidural blocks' tendency to take longer to set7. 2-CP has a quick onset of action and a good sensory and motor block. Because of its limited protein binding and quick metabolism by pseudo-cholinesterase, 2-CP has a shorter duration of action.8-11 Several previous research has raised concerns about the safety and potential neurotoxicity of 2-CP with preservative.12, 13 In our study, n=80 were enrolled patients and randomly assigned in group B, were received 2ml of 0.5% hyperbaric bupivacaine and Group C were received 2.5 ml of preservative-free 1% 2-Chloroprocaine which shows, Group C has good effects on the onset of a sensory block with (2.01±10.9 min) as p<0.001. As the onset of motor block was seen to be (3.71±1.254 min) as p<0.001 respectively. As the main characteristics were seen to found highly improved condition as mean time to achieve the maximum level of a sensory block with (3.01±0.92 min) and complete duration of motor block is seen to be (98.27±28.3 min). As the duration of analgesia in minutes was seen to be (109.32±25.8 min) as p<0.001 with p<0.05 respectively. As the result with Group C receiving 2.5 ml of preservative-free 1% 2-Chloroprocaine when compared with Group B receiving 2ml of 0.5% hyperbaric Bupivacaine found to be both clinically and statistically significant with the study carried out by Lacasse M. A et. al 14 who compare 2-CP with Bupivacaine for spinal anaesthesia in an elective ambulatory setting in n=106 patients. The average time to discharge was 277 min in the 2-CP group and 353 min in the Bupivacaine group, a difference of 76 min (95% confidence interval [CI]: 40 to 112 minutes; p<0.001) with p-value <0.05. The average time for the complete sensory block regression was 146 min in the 2-CP group and 329 min in the Bupivacaine group, a difference of 185 min (95% CI: 159 to 212 min; p<0.001) with a p-value <0.05. Times to ambulation and micturition were also significantly lower in the 2-CP group. Spinal anaesthesia with 2-Chloroprocaine provides both adequate duration as well as the depth of surgical anaesthesia for short procedures with the advantages of faster spinal anaesthesia resolution and early discharge from hospital compared with spinal anaesthesia with Bupivacaine. In a similar study carried by Ashwini S et al.,15 the mean duration of sensory block was (61.83 ± 23.54 min) for group CP, was shorter than group B which had (174.67 ± 41.17 min) and it was statisticall significant, p<0.001 with p<0.05. Group CP had a very less incidence of hypotension (30% Vs 55.33%) compared to group B. When compared to low-dose Bupivacaine for an uncomplicated elective LSCS, chloroprocaine for a subarachnoid block is a safer and more appropriate option for the patient. The use of intrathecal opioids in caesarean section spinal anaesthesia improves spinal block and provides good and long-lasting postoperative analgesia. Some of the negative effects of spinal anaesthesia can be reduced by lowering the dosage of LA administered. These include maternal hypotension, high spinal block, and prolonged motor block. In the current study, the onset time of the sensory block of Group C is (2.01 ± 1.09 min) in comparison to Group B which is (3.36 ± 1.2 min). The onset time of motor block was (3.71 ± 1.25 min) in Group C when compared to Group B is (as p<0.001 with p-value <0.05). The onset of sensory blockade was significantly faster in Group B (76.74±11.94 min) compared to Group B (168.60 ± 12.41min) as p<0.001 with p-value<0.05. The time of onset of the motor block was faster in Group B which is statistically significant (P < 0.05). The time duration of the motor block was shorter significantly in Group B (98.27 ± 28.3 min) as compared to Group C (164.82 ± 23.47 min) as p < 0.001 with p<0.05. Time duration of analgesia was shorter significantly in Group B (109.32 ± 25.8 min) when compared to Group C (189.28 ± 23.47 min) as p<0.001 with p-value <0.05 respectively. The occurrence of hypotension in Group C is lesser compared to Group B as n=8 (20%) and n=11 (27.5%) respectively. The results are similar to Jain N et al.,16 100 patients were equally divided into Group A (n = 50) and Group B (n = 50). Group A subjects were given SAB with isobaric 1% 2-Chloroprocaine 5 ml (50 mg) and Group B (n = 50) were given SAB with 0.5% hyperbaric Bupivacaine 2 ml (10 mg). The onset of sensory block was fast in Group A significantly (1.66 ± 0.49 min) as compared to Group B (3.00 ± 0.58 min) (P < 0.05). The total sensory block duration was shorter in Group A (P<0.05) significantly. The regression time for two-segments was also faster in Group A (41.44 ± 5.41 min) which was significant, as compared to Group B (70.24 ± 10.38 min) (P < 0.05). The onset time of the motor block in Group A was significantly faster (P < 0.05). Total motor block duration in Group A was shorter significantly (95.7 ± 9.8 min) when compared to Group B (186.26 ±13.5 min) (P < 0.05). The total duration of spinal analgesia in Group A was shorter significantly (97.22 ± 11.82 min) when compared to Group B (191.58 ± 37.06 min) (P<0.05). The improved operating environment would result from a faster onset time of the motor block, which would be especially beneficial for parturients receiving LSCS who need a quicker induction of anaesthesia. 2-chloroprocaine (2-CP) showed faster offset times to the end of anaesthetic, supported early ambulation, and hospital discharge as compared to Bupivacaine. These findings suggest that 2-CP could be a viable alternative to low-dose long-acting local anaesthetics in ambulatory surgery. 17We also noted that the duration of analgesia with Group C is (109.32 ± 25.8 min) when compared to Group B is (189.28 ± 39.76) as p<0.001 with p-value<0.05. The result was seen both clinically and statistically significant. Minor side-effects such as hypotension in n=8 subjects (20%) followed by bradycardia n=2 (5%), vomiting with n=3 (7.5%) and least with nausea with n=2 (5%) respectively. The result as Group C is significant when compared to Group B. The present study shows relevance with a study Sathyanarayana V et al.,18 noted that the duration of analgesia was more in the bupivacaine group (168.41 ± 37.94 min) as compared to the chloroprocaine group (70.58 ± 31.15 min) and the difference was significant statistically. Common side effects such as hypotension, bradycardia, nausea, vomiting were noted in both groups and the difference was statistically not significant. Chloroprocaine appears as an alternative to Bupivacaine for a subarachnoid block in uncomplicated elective lower segment caesarean section patients. Breastfeeding is known to be affected by Caesarean birth in a variety of ways, including a decrease in the commencement of breastfeeding, a decrease in the incidence of exclusive breastfeeding, a considerable delay in the onset of lactation, and an increase in the chance of formula supplementation. More rapid reversal of motor blockade could lessen the time spent in the PACU, improving breastfeeding initiation and reducing mother–newborn separation. Despite the fact that 2-CP has a favourable pharmacokinetic profile, resulting in a rapid onset of action and more predictable motor block regression, additional research is needed to prove a shorter stay in the PACU.19
CONCLUSION Our study revealed that intrathecal preservative-free 1% 2-chloroprocaine is a safe and effective option compared to intrathecal bupivacaine in elective LSCS, as it provides quick onset of anaesthesia, a predictable sensory block height, acceptable duration of motor block, and adequate analgesia. for sufficient duration.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE We further confirm that any aspect of the work covered in this manuscript that has involved human participants has been conducted with the ethical approval. HUMAN AND ANIMAL RIGHTS No animals were used for studies that are base of this research. The study on humans was conducted in accordance with the ethical rules of the Helsinki Declaration and Good Clinical Practice.
ACKNOWLEDGEMENTS We explicit our heartfelt gratitude to our honourable Principal, Belagavi Institute of Medical Sciences, Belagavi, teaching and non-teaching faculty members of Department of Anaesthesiology and also, all the subjects enrolled in our study for their valuable time and co-operation without which this study would not have been feasible.
REFERENCES
Policy for Articles with Open Access
|
|
 Home
Home