Official Journals By StatPerson Publication
|
Table of Content - Volume 3 Issue 1 -July2017
Study of comparative evaluation of bupivacaine and bupivacaine with dexmedetomidine in subarachnoid block for below umbilical surgeries
Nitinkumar Ingle1*, Pradnya Hingole2, Deepak kokane3, Satish G Deshpande4
1SMO, LTMMC, Sion, Mumbai, Maharashtra, INDIA. 2Fellow, IDCCM Fortis Hospital, Mulund, Mumbai, Maharashtra, INDIA. 3Associate Professor, 4Professor and HOD, Department of Anaesthesia, Government Medical College, Latur, Maharashtra, INDIA. Email: nbi188@gmail.com
Abstract Background: Spinal anaesthesia is the most preferred regional anaesthesia technique as it is easy to perform, economical and produces rapid onset of anaesthesia and complete muscle relaxation. Dexmedetomidine, an α2 agonist drug providing stable hemodynamic conditions, good quality of intraoperative and prolonged postoperative analgesia with minimal side effects. Aim and Objective: To compare and evaluate bupivacaine and bupivacaine with dexmedetomidine in subarachnoid block for below umbilical surgeries. Material and Methods: It was prospective, randomized, double blind study conducted at tertiary care centre over a period extending from January 2014 to October 2015. The study included a total of 100 patients divided into two groups (each of 50 patients) i.e. control and study groups depending upon drugs administered. Results: The onset of sensory block as well as time for attaining maximum sensory level was significantly faster (p < 0.05) in study group as compared to control group. Also, regression of sensory block was slower and onset of motor block is quicker in patients those who received intrathecal dexmedetomidine. There was a significant prolonged duration of sensory analgesia among study group. Summary and Conclusions: Use of0.5% hyperbaric Bupivacaine (15mg) with dexmedetomidine (5mcg) in subarachnoid block Leads to significantly quicker onset of Motor and Sensory block also a minimal intraoperative and postoperative complication as compared to 0.5% hyperbaric Bupivacaine. Key Words: bupivacaine, dexmedetomidine.
INTRODUCTION Since the introduction of spinal anaesthesia in 1898 by Dr. August Bier, who described the intrathecal administration of cocaine, spinal anaesthesia is preferred over general anaesthesia, particularly in surgical procedures of lower abdomen and lower limbs.1Its easy to perform, economical and produces rapid onset of anaesthesia and complete muscle relaxation. The aim of intrathecal local anaesthetic is to provide adequate sensory and motor block necessary for all below umbilical surgeries. Hyperbaric bupivacaine is the most commonly used intrathecal local anaesthetic.2,3 A common problem during lower abdominal surgeries under spinal anaesthesia is visceral pain, nausea, and vomiting.4Dexmedetomidine, a new highly selective α2-agonist is the S-enantiomer of medetomidine, a substance that has been used for sedation and analgesia in veterinary medicine for many years. It is α2 agonist drug providing stable hemodynamic conditions, good quality of intraoperative and prolonged postoperative analgesia with minimal side effects.Intrathecalα2 receptor agonists have been found to have antinociceptive action for both somatic and visceral pain.5 The clinical studies about the use of intrathecaldexmedetomidine in surgical patients are limited in the literature; hence to contribute the literature and to see whether it alleviates the side effect of clonidine and midazolam, we decided to study the efficacy and safety profile of dexmedetomidine in combination with local anaesthetic in subarachnoid block for below umbilical surgeries.
MATERIAL AND METHODS The present study was conducted at tertiary care centre over a period extending from January 2014 to October 2015. It was conducted after approval frominstitutional ethics committee and written, valid, informed consent of all the patients. It was prospective, randomized, double blind study carried out to evaluate the efficacy of dexmedetomidine as an adjuvant to intrathecal 0.5% Bupivacaine heavy for below umbilical surgeries. The study included a total 100 patients belonging to ASA grade I and II of either sex with age between 15-45 years posted for elective below umbilical surgery (ASA-American Society of Anaesthesiologists). Out of the hundred patients included in this study, 50 patients of each group scheduled for below umbilical surgeries. They were randomised by computer generated random number sequence and sealed envelope technique into two groups. Patients included in this study underwent thorough preoperative evaluation on day before surgery. Patients those who consented for study were taken in operation theatre and procedure of spinal anaesthesia explained to the patient. All monitors attached and preoperative basal pulse rate, systolic and diastolic blood pressure, respiratory rate and SpO2 were noted. On successful lumbar puncture drug injected slowly into subarachnoid space by all aseptic precautions as per group allotment. The total volume injected was 3.5 ml in both the groups. Patients were divided into two groups (each of 50 patients) i.e. control group (Group B) and study group (Group BD) depending upon drugs used. GROUP B: (control group): Received inj. Bupivacaine 0.5 % (heavy) 15 mg (3cc) + Normal saline 0.5 cc GROUP BD: (study group): Received inj. Bupivacaine 0.5 % (heavy) 15 mg (3cc) + inj.dexmedetomidine (5mcg) 0.5cc Inclusion Criteria
Exclusion Criteria
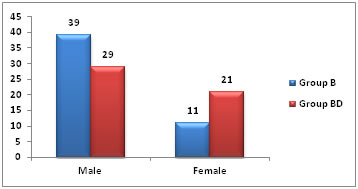
Pulse rate, systolic and diastolic blood pressure, respiratory rate and SpO2 were monitored intraoperatively and in postoperative period for 9 hours after spinal anaesthesia. Other parameters observed i.e. sensory and motor block parameters, analgesia time and VAS score and subjected to statistical analysis. Analgesia was defined as loss of the sensation to pinprick and anaesthesia as loss of sensation to touch. Motor blockade was assessed by straight leg raising while lying supine and was graded according to Modified Bromage Scale.6 RESULTS AND OBSERVATIONS In the present study, among Group B, there were 39 male and 11 female, while in Group BD, there were 29 male and 21 were female (Table No. 01).
Table 1: Gender Wise Distribution of patients
Figure 1: Gender Wise Distribution of patients From Table No 02, among Group B mean age of patient was 34±8.7 years. The mean height was 154.2±15.04 cm and the mean weight was55.04±8.80 kg. Highest age was 48 years and lowest age was 19 years, highest height was 189 cm and lowest was 126 cm, highest weight was 78 kg and lowest weight was 44 kg. In Group BD mean age of patient was 32.9±8.00 years. The mean height was153.23±14.31 cm and the mean weight was 54.02±8.22 kg. Highest age was 45 years and lowest age was 18 years, highest height was 189 cm and lowest was 127 cm, highest weight was 72 kg and lowest weight was 44 kg. Table 2: Patients Characteristics among Two Groups
The present study was undertaken to evaluate the effect of addition of dexmedetomidine in subarachnoid block along with 0.5% Bupivacaine (H) on sensory, motor, hemodynamic and other analgesic parameters both intraoperatively and postoperatively. From Table No. 03 below, theonset of sensory block as well as time for attaining maximum sensory level was significantly faster (p < 0.05) in study group as compared to control group. Also, its clear that regression of sensory block was slower and onset of motor block is quicker in patients those who received intrathecaldexmedetomidine. There was significant prolonged duration of sensory analgesia in dexmedetomidine group i.e. in Group BD as compared to control group (p<0.001).
Table 3: Variation of various anaesthetic parameters among two study groups
In our study, intraoperative andpostoperative complications observed were inadequate level of analgesia, bradycardia, tachycardia, hypotension, high level of block. We found that there was no statistically significant difference of intraoperative and postoperative complications between both the groups as the p value > 0.05. DISCUSSION Dexmedetomidine hydrochloride, a newer agent within the class of α2 adrenoreceptor agonist delivers clinically effective sedation with analgesic property for use in intensive care unit setting. Additionally, it has an ability to eliminate or reduce the need for other analgesic medications. It was introduced in clinical practice in the United States in 1999 and approved by the FDA only as a short-term (<24 hours) sedative for mechanically ventilated adult ICU patients.7 The dose of dexmedetomidine used in subarachnoid block ranged 3-5 µg in various studies showing effective clinical and safety profile. Hence, in this study, we used 5 µg preservative free dexmedetomidine with 15 mg of hyperbaric bupivacaine intrathecally in Group BD. The demographic data such as age, sex, height and weight being comparable has no influence on outcome of the study. In present study mean time for onset of sensory block was 2.47±0.29 min in Group BD, which was quicker as compared to 4.32±0.61 min in GroupB. Onset sensory block was significantly faster (p < 0.05) in study group ascompared to control group. The findings of this study are similar to other studies i.e. quicker onset of sensoryblock in patients of dexmedetomidine group comparable with R. Brinda et al8, Memon N et al9,Chatrath V et al10. Our findings were contradictory to study conducted by Feroz Ahmad Dar et al11and Sangeeta Agarwal Bansal et al12who had found no difference in time of onset using 5 µg dexmedetomidine. In our study time to achieve maximum sensory block was 5.39±0.31min in Group BD as compared to 7.51±0.27min in Group B. Time to achieve maximum sensory block was significantly lower (p value<0.05) in dexmedetomidine group as compared with control group. It indicates cephalad spread of sensory block occur faster when dexmedetomidine was added to intrathecal Bupivacaine. This finding was similar to that of study carried out by R. Brinda et al8 while, it was contradictory to findings of Sangeeta Agarwal Bansal et al12andFeroz Ahmad Dar et al11, Hala E A Eid et al13 who found no such difference regarding timeto achieve maximum sensory block. About motor block parameters, mean time of onset of motor block was significantly quicker (p<0.001) i.e. 3.53±0.27 min in Group BD as compared with 5.87±0.29 min in Group B. It was comparable to study carried out by R. Brinda et al8 Who found mean time of onset of motor block 2.30±0.45 min in dexmedetomidine group (5mcg) as compared to 6.57±0.49 min in control group. Regarding mean duration of motor blockade in Group BD i.e. dexmedetomidine group was 227.1±1.76 min which was significantly prolonged (p<0.001) as compared with Group B. It wascomparable with study carried out by Chatrath Vet al10who found that the mean duration of motor blockade was 318.36±9.374 min in dexmedetomidine group as compared to only 146.94±9.713 min in control group (p<0.05). Similar results were also obtained by Sunil B.V. et al14 After analyzing the data collected by using chi-square test, there was no statistically significant difference of intraoperative and postoperative complications between both the groups as the p value > 0.05. Results of our study are similar with the studies carried out by Sunil B.V. et al14, ChatrathV et al10, Memon N et al9, R. Brinda et al8in respect of onset of sensory block, onset of motor block, duration of sensory block, duration of motor block, duration of analgesia, intraoperative and postoperative complication.
SUMMARY AND CONCLUSIONS The demographic data such as age, sex, height and weight were comparable in both groups and has no influence on outcome of the study. Use of0.5% hyperbaric Bupivacaine (15mg) with dexmedetomidine (5mcg) in subarachnoid block
And is useful in subarachnoid block for below umbilical surgeries where prompt onset and prolonged duration of post-operative analgesia is needed.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home