Official Journals By StatPerson Publication
|
Table of Content Volume 6 Issue 1 - April 2018
A prospective study of comparison of control of bleeding with tranexamic acid vs placebo in acute trauma patients
G R Rajashree1, Anuradha Swaminathan2*
1,2Professor, Institute of Critical Care and Department of Anaesthesiology, Rajiv Gandhi Government General Hospital and Madras Medical College, Chennai, Tamil Nadu, INDIA. Email: dr.rajashree@hotmail.com
Abstract In our trauma care centre we receive around 2000 cases per month ranging from Polytrauma, Faciomaxillary injury, Both bone fracture Upper limb, Lower limb, Vascular trauma, Abdominal injuries of patients of variable age groups. Methods: 30 patients of each group were chosen with the various trauma mentioned above. We started the Randomised Prospective Comparative Clinical study with Tranexamic acid 1 gram Intra Venously in 100 ml of Normal saline within 30 minutes of the arrival at trauma ward. The other group had 100 ml of Normal Saline. The case chosen were between 20 to 60 years of age both male and female. Inj. Tranexamic acid group Vs control group were chosen with similar condition like Right facial trauma Vs Left facial trauma. Conclusion: We conclude that infusion of Tranexamic acid reduces intraoperative blood loss (P = 0.003). Key Words: Trauma, Tranexamic acid, blood transfusion, blood products.
INTRODUCTION A Road Traffic Accident (RTA) can be defined as, ‘An event that occurs on a way or street open to public traffic; resulting in one or more persons being injured or killed, where at least one moving vehicle is involved. RTA is a collision between vehicles; between vehicles and pedestrians; between vehicles and animals; or between vehicles and geographical or architectural obstacles.’ Road traffic accidents are a human tragedy. They involve high human suffering and socioeconomic costs in terms of premature deaths, injuries, loss of productivity, and so on.1 If no action is taken, road traffic crashes are predicted to result in the deaths of around 1.9 million people annually by 2020.2 Hence the goal of the United Nations’ Decade of Action for Road Safety 2011- 2020 is to save five million lives.3 According to the World Health Organization (WHO), road traffic injuries are the sixth leading cause of death in India with a greater share of hospitalization, deaths, disabilities and socio-economic losses in the young and middle-aged population.4 Road traffic injuries also place a huge burden on the health sector in terms of pre-hospital and acute care and rehabilitation.5
Table 1:
Tranexamic Acid: Tranexamic acid (TXA) is a long-established antifibrinolytic drug that was developed in Japan in 19656,7. Historically, it is commonly used for a reduction of the blood loss in perioperative situations including cardiac, orthopedic, oral, gynecological, and urological surgeries8-13. Several meta-analyses elucidated the efficacy of TXA on the blood transfusion requirements14,15. In 2010, the results of the Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage 2 (CRASH-2) trial, the first multicenter randomized, placebo-controlled trial evaluating the effects of TXA in patients with trauma, were published in Lancet16. After the launch of its sensational results, the main stream treatment protocol in trauma changed worldwide to include TXA administration17,18. However, unrestricted usage of TXA has been criticized and reconsidered since several studies have pointed out its potential detrimental effects].
Figure 1 Figure 2 Figure 3
TXA is a synthetic derivative of the amino acid lysine that inhibits fibrinolysis22 Plasma plasminogen is activated and 19-21 Converted to plasmin by t-PA in the presence of fibrin. Plasmin mainly degrades fibrin into fibrin/fibrinogen degradation products. The degradation process requires the connection of the lysine binding sites of plasminogen with the lysine residues on the surface of fibrin. Since TXA has a high affinity for the lysine binding sites of plasminogen, it blocks the interaction of plasminogen with the lysine residues of fibrin and exhibits an antifibrinolytic effect22 Because the development of DIC associated with the fibrinolytic phenotype may increase the mortality in trauma, TXA is potentially beneficial to patients who have developed hemostatic abnormalities during the early phase of trauma. On the other hand, a delayed increase in PAI-1 results in the inhibition of fibrinolysis in the later phase23,24. Administration of TXA could accelerate this change and develop detrimental effects when it is used during the fibrinolytic shutdown phase. In fact, numerous basic research studies have demonstrated the pro-thrombotic state enhanced by TXA administration25-28. That is, the estimation of the coagulation/fibrinolysis status is quite important to gain the greatest benefit from TXA administration in patients with trauma.
MATERIAL AND METHODS The study was conducted in Rajiv Gandhi Government General hospital, Madras Medical College, Chennai after a pilot study of 10 cases in each group. After obtaining approval from the Institutional Ethics Committee. Written informed consent was obtained from the patient and relatives. The patients included were 20 to 60 years, Glasgow Coma Scale (GCS) 15/15, Body Mass Index <40, who have given valid informed consent with similar injuries like Polytrauma, fracture mandible/zygomatic process, long bone fracture upper limb, long bone fracture lower limb, lacerated wound. The patients excluded were unconscious patients with severe head injury, bleeding disorders and Coagulation abnormalities, hemodynamically unstable, seizure disorder, patients with severe cardiovascular, endocrine, respiratory, renal, hepatic, psychiatric diseases, pregnant and lactating mothers. Monitors included were Electocardiography (ECG), Non-invasive Blood Pressure (NIBP), Pulseoximetry. After randomization, patients with similar pathology were allocated into two groups (Group T – Tranexamic acid and Group C- Control). After taking blood samples for routine investigations, Inj. Tranexamic Acid 1 gm intravenously in 100 ml of Normal Saline (NS) over a period of 20-30 minutes and the other group received 100 ml of NS. They were monitored with Heart Rate (HR) and Blood pressure (BP) at 30 mins, 1st hour, 2nd hour, 4th hour and 6th hour. The patients were taken up for surgery within g hours of admission. The blood loss was estimated in the perioperatively and the data was collected regarding the transfusion throughout the period. The collected data were analysed with IBM.SPSS statistics software 23.0 Version which includes tests like Shapiro Wilk’s test, Unpaired sample t-test, ANOVA and Chi-Square test was used.
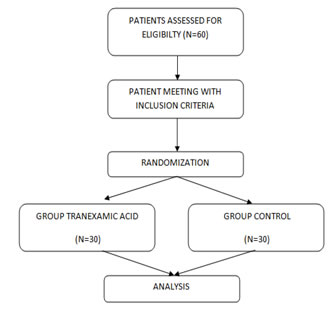
RESULTS Out of 60 patients assessed for eligibility, they were randomized according to the study protocol. Figure1 shows the consort diagram of the enrolled patients. In all the statistics, the probability value .05 is considered as significant level.
Figure 1: Consort flow chart
Table 1: Age Distribution
The age range distribution between the Groups T and C shows there is no statistical significant Chi -Square value is 7.042 with ( P = 0.134).This reveals that age is equally distributed in both the groups. Table 2: Sex Distribution
The gender distribution between the Groups T and C shows there is no statistical significant Chi -Square value is 1.27 with (P = 0.260).This reveals that gender is equally distributed in both the groups
Table 3: Variability of Heart rate in both groups
Table 4: Changes in systolic blood pressure
Table 5: Changes in diastolic blood pressure
The comparison between Group T and C in diastolic blood pressure shows no statistical significance in all the time period.
Table 6: Blood transfusion requirement
The need of transfusion between the Groups T and C shows that there is highly statistical significant Chi -Square value is 8.53 with (P = 0.003). This reveals that need of transfusion is very less in T groups (6 numbers) than the C group (17 number).
Table 7: Amount of Blood transfused
The units of blood transfused between the Groups T and C shows that there is statistical significant Chi -Square value is 8.86 with (P = 0.032).This reveals that no transfusion is very high (24 numbers) in T groups than C group (13 number). Table 8:
The comparison was done between time periods in Group T and C separately, it was found that there was no statistical significance in heart rate and DBP and significant in SBP whereas in C group, highly significant in heart rate but significant in SBP and DBP.
DISCUSSION As an antifibrinolytic lysine analogue TXA binds to plasminogen and plasmin, the central enzyme in fibrinolysis. It inhibits plasminogen activation but does not specifically impair the enzymatic activity at the catalytic site. It is well accepted that TXA exerts an antifibrinolytic action to reduce blood loss, In addition to its role in fibrinolysis, plasmin activates and inactivates a number of important procoagulant and anticoagulant molecules and interacts with cellular components involved in the hemostatic balance. Plasmin also plays a role in inflammation, angiogenesis, and wound healing, and TXA has been shown to have beneficial effects with respect to inflammatory and other responses following ischemia reperfusion. It is not possible from available data to identify specific mechanism(s) of action that are involved in potential salutary or deleterious effects of TXA in trauma. As pointed out by others, mechanisms other than inhibition of fibrinolysis may be involved. Thepotential importance of the complement system and impact of immune modulation suggest that an inflammatory pathway may be involved in the observed effects of TXA.
smaller effect on all-cause mortality, although a reduction in the hazard of death due to bleeding is evident forseveral days. Whilst the benefit of early TXA treatment is greateston the day of injury, the adverse effect of late administration manifests as an increased risk of death due tobleeding over subsequent days. late initiation of TXA treatment may increase the risk of thrombotic disseminated intravascularcoagulation (DIC).By inhibiting fibrinolysis, TXAmight increase the risk of DIC. Although the underlyingpathology in DIC is thrombosis, due to the consumptionof coagulation factors DIC often manifests as bleeding.Among patients in whom treatment is initiated beyond3 h of the injury, deaths apparently due to bleeding mayhave been due to thrombotic DIC. Based on WHO mortality data and a systematic review of the literature we estimate that there are about 400,000 in-hospital deaths from bleeding each year worldwide. If all hospitalised bleeding trauma patients could be treated with TXA within an hour of injury then up to 128,000 of these premature deaths could be averted. If they could be treated within three hours of injury then up to 112,000 premature deaths could averted. Although there is considerable uncertainty in the estimates even most conservative suggest that tens of thousands of deaths could be averted. TXA is a practicable treatment suitable for use in a range of health-care settings, including pre-hospital. If TXA was used in the pre-hospital setting then many more premature deaths might be averted. In our study, age and gender are equally distributed in both the groups. Heart rate shows statistical significance in all the time period except in 4th hour. The need of transfusion between the Groups T and C shows that there is highly statistical significant Chi -Square value is 8.53 with (P = 0.003).This reveals that need of transfusion is very less in T groups ( 6 numbers) than the C group (17 number). The units of blood transfused between the Groups T and C shows that there is statistical significant Chi -Square value is 8.86 with (P = 0.032).This reveals that the number of no transfusion is very high (24 numbers) in T groups than C group (13 number). When the comparison was done between time periods in Group T and C separately, it was found that there was no statistical significance in heart rate and DBP and significant in SBP whereas in C group, highly significant in heart rate but significant in SBP and DBP. The group which had IV Tranexamic acid had less bleeding, less transfusions during the perioperative period when compared to the placebo group.
CONCLUSIONS Tranexamic acid is found to be promising drug to improve survival after traumatic hemorrhage. Knowledge gap still remains, and our ability to provide additional information will allow the trauma community to refine and improve its ability to apply TXA in the safest and most effective manner. The prioritization of knowledge gaps is based on acute care trauma centres, which receive more trauma cases on a day today basis. At individual centers, expanded monitoring and laboratory analyses will provide more information. At the larger scale, a targeted, prioritized research effort is needed to address key knowledge gaps and contribute to refinement of practice guidelines over time.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home