Official Journals By StatPerson Publication
|
Table of Content Volume 6 Issue 1 - April 2018
A prospective randomised double blind clinical comparative study of ramosetron hydrochloride and dexamethasone versus ondansetron hydrochloride and dexamethasone in prevention of post operative nausea and vomiting in patients undergoing gynaecological surgeries under spinal anaesthesia
Mohsin Wazir1*, Faizan I Asrar Nazki2
1Registrar, Department of Anaesthesiology, Yashoda Super speciality Hospital, Malakpet, Hyderabad, 500036, INDIA. 2PG Resident, Department of Biochemistry, Owaisi Hospital and Research Centre, Santoshnagar, Hyderabad-500008, INDIA. Email: mohsinwazir@gmail.com
Abstract Background: Postoperative nausea and vomiting (PONV) is one of the most unpleasant and distressing symptoms which follow anaesthesia and surgery. Patients undergoing gynaecological surgery have been associated with high risk for developing PONV. The anaesthetic consequences are aspiration pneumonitis and discomfort in recovery. Of the many different modes of intervention to prevent PONV, antiemetic drugs play an important role in therapy of PONV. Presently, there is no single PONV antiemetic medication or technique that is 100% effective for all patients and a search for better drug continues. Combination of 5HT3 receptor antagonists and Dexamethasone has been recommended for prophylaxis in patients at risk of PONV. Objectives: To compare the effectiveness of combination of Ramosetron 0.3mg and Dexamethasone 8mg v/s Ondansetron 4mg plus Dexamethasone 8mg in prevention of postoperative nausea and vomiting. To evaluate the effectiveness of combination of Ramosetron 0.3mg and Dexamethasone 8mg in prevention of post operative nausea and vomiting. In patients undergoing elective gynaecological surgeries under spinal anesthesia. Material and Methods: A prospective, randomized, double blinded comparative study of Ramosetron hydrochloride and Dexamethasone versus Ondansetron hydrochloride and Dexamethasone in prevention of post operative nausea and vomiting in 60 ASA 1 and 2 patients divided in two groups undergoing gynaecological surgeries. Group Ond+ Dexa [n=30] received Ondansetron (4mg) + Dexamethasone (8mg) while as Group Ram + Dexa [n=30] were given Ramosetron (0.3mg) +Dexamethasone (8mg).The study was conducted in Yashoda super speciality Hospital Malakpet Hyderabad from 1st June 2015 to 31st May 2016. The incidence and severity of Post operative nausea and vomiting was studied for period of 24 hrs. Severity was assessed using PONV Score [0=no nausea; 1=nausea; 2=retching; 3=vomiting]. The need for rescue antiemetics and adverse effects were also studied. Results: In our study in 24 hour period, complete response in Group O was 46%, Group R was 83%. Overall incidence of nausea in Group O was 46% and Group R was 16%. Overall incidence of retching in Group O was 16% and Group R was 3%. Overall incidence of vomiting in Group O was 10% and Group R was 0% respectively. The requirement of rescue antiemetics in Group O and Group R was 33%and 3 % respectively. Conclusion: The study suggested combination of Dexamethasone (8mg) + Ramosetron (0.3mg) is a better alternative to combination of Dexamethasone (8mg) + Ondansetron (4mg) in preventing PONV in high risk patients. Key Words: PONV; Ondansetron; Ramosetron; Dexamethasone; Total Abdominal Hysterectomy.
INTRODUCTION Postoperative nausea and vomiting (PONV) is one of the most unpleasant and distressing symptoms which follow anaesthesia and surgery and lead to serious postoperative complications.1 Postoperative nausea and vomiting, commonly abbreviated PONV, is defined as nausea and or vomiting that occurs within 24 hours after surgery and can occur following general, regional or local anaesthesia.2 PONV has been a potential complication following surgery and anaesthesia since the “ether” era, with an occurrence of 75% to 80% at that time.3 The overall incidence of PONV has been reported to be between 20% and 30%, but can increase up to 80% in high risk patients.1,4 Postoperative nausea and vomiting is often referred to as the “big, little problem” within the anaesthesia world and has been a common complication for both in-patients and out-patients undergoing virtually all types of surgical procedures.5,6 PONV occurs frequently in gynecological, obstetric, ocular, breast and middle ear surgeries7 Patients undergoing major gynaecological surgeries are especially prone to PONV, with reported incidence of 50-75%.7,8 A number of factors influence the occurrence of PONV. Patient factors like age, female gender, nonsmoker, obesity, anxiety, history of motion sickness or previous PONV and gastro paresis, operative procedures, anaesthetic techniques like drugs for general anaesthesia, regional anaesthesia and monitored anaesthesia care, and post-operative factors like pain, ambulation, oral in-take and opioids, determine the incidence of PONV.7,8,9 The consequences of PONV are surgical, physical and anaesthetic complications for patients and financial implications for the hospitals or institutions. Surgical consequences include disruption of vascular anastomoses and increased intracranial pressure.10 Physical consequences include sweating, pallor, tachycardia, pain abdomen, increased chances of oesophageal tear, wound dehiscence and electrolyte imbalance.[10] The anaesthetic consequences are increased aspiration pneumonitis and discomfort in recovery. For institutions there is increased financial burden because of increased nursing care, delayed discharge and unexpected admissions. In ambulatory surgery too, PONV delays the hospital discharge. This necessitates the use of prophylactic antiemetics.11 None of the available antiemetics are entirely effective for preventing PONV, especially in high-risk patients.12 The management of PONV has improved greatly in recent years, with the introduction of 5-Hydroxytryptamine (5-HT3) receptor antagonists and are widely regarded as the most efficacious antiemetics available today and are currently recommended as the agents of first choice to control PONV in most instances.12 Since at least four major receptor systems are involved in the etiology of PONV, a better prophylaxis might be achieved by using a combination of agents acting at different receptor sites.12 For example, if the serotonin receptors have already been blocked, consider adding an anticholinergic, antidopaminergic or antihistaminic. The concept of combination antiemetic therapy was first introduced in chemotherapy induced vomiting. Its success prompted similar research in the field of PONV.12 There is increasing evidence that the multimodal approach may improve the outcome. Double and triple antiemetics combinations are recommended for patients with high risk for PONV.11,12 Several studies are being conducted with different drug combinations and different dosages. Combination of 5HT3 receptor antagonists and Dexamethasone has been recommended for prophylaxis in high risk group.13 The most common prophylactic antiemtic combination used to prevent PONV in our institution is a combination of Intravenous Ondansetron, a 5HT3 receptor antagonist with Dexamethasone. Ramosetron is a newly introduced 5HT3 receptor antagonist with potential advantage of greater efficacy with prolonged duration of action. (Elimination half-life of Ramosetron is 9 hr).14 It has been introduced in India only in the year 2011 and not many studies have been done using this drug for PONV in India. Hence the present study was designed to assess the efficacy and safety of Ramosetron versus Ondansetron in combination with Dexamethasone in preventing and reducing the incidence of PONV after elective abdominal hysterectomy performed under spinal anaesthesia. MATERIAL AND METHODS A prospective, randomized double blinded comparative study of Ramosetron hydrochloride and Dexamethasone versus Ondansetron hydrochloride and Dexamethasone in prevention of post operative nausea and vomiting on 60 ASA class I/II patients posted for elective gynaecological surgeries under spinal anaesthesia was conducted in the department of anaesthesiology, Yashoda super speciality Hospital Malakpet Hyderabad from 1st June 2015 to 31st May 2016. After appropriate institutional human research scientific committee and ethics committee approval and written, informed consent of patient after explaining them about the study in the language they understand.60 normal adult female patients aged between 30-60 years with ASA class I and II were enrolled into the study in our hospital. Only patients undergoing elective gynaecological surgeries under spinal anaesthesia were enrolled in this study. Patients with known hypersensitivity or contra-indications to study drug, patients with history of nausea, vomiting or retching in 24 hours before anaesthesia, patients who received anti-emetic drugs or drugs with anti-emetic property during hours before anaesthesia, patients with diabetes mellitus, patients on chronic opioids use, patients with history of motion sickness, pregnant patients, Epileptic patients, patients with history of post operative nausea and vomiting in previous anaesthetic exposure, patients with significant cardiac, pulmonary, hepatic or renal dysfunction and patients having contraindications for spinal anaesthesia were all excluded from the study. The study population randomly assigned to two groups with thirty patients in each group received the following prophylactic anti emetic combination therapy. Group Ond + Dexa [n=30]: Dexamethasone (8mg) + Ondansetron (4mg). Group Ram + Dexa [n=30]: Dexamethasone (8mg) + Ramosetron (0.3mg). Pre anaesthetic evaluation was done on the previous day of surgery and patients were assessed for risk factors for PONV. Written informed consent was taken from all patients selected for the study. A thorough history taking and general and systemic examination was done. Basic laboratory investigations (Hemoglobin level, total count and differential count, urine routine, and screening of chest x-ray, ECG, RBS, blood urea, serum creatinine and thyroid function tests) were evaluated. Patients were advised to remain nil orally for solids after 12 pm and 2 hours for clear fluids. All of them received tabl et al prex 2.5mg and Ranitidine hydrochloride 150mg orally on the night before surgery. On arrival to operation theatre, routine monitors (electrocardiogram, pulse oximetry, NIBP) were connected and basal vital parameters were recorded. An 18G intravenous cannula was secured and an intravenous infusion of 500ml (10-15ml/kg) of Ringer’s lactate was administered before induction of spinal anaesthesia. Patients were placed in the left lateral or sitting position and Subarachnoid block was performed in the L2-3 or L3-4 interspace using a midline approach with 25G Quincke’s spinal needle. After confirming a free flow of cerebrospinal fluid, 2.5ml of 0.5% Bupivacaine heavy and 0.5ml of Fentanyl (25mcg) was injected. After injection of the anaesthetic solution, the patient was turned to supine position. Time of onset of action up to T6 level was noted using pin-prick method before surgical incision, and surgery was allowed to commence after 5 minutes. Supplemental oxygen 5L/min was administered via M C face mask during anaesthesia and surgery. Any patients having inadequate block, requiring supplemental analgesics or general anaesthesia and patients who had episodes of severe hypotension were dropped from the study. Intraoperatively, non-invasive blood pressure measured by an automated cuff blood pressure monitor, continuous pulse oximetry and electrocardiograph monitoring were done using multi parameter. Estimated fluid deficit and maintenance fluid requirements were infused as required during the case. Duration of surgery was noted. Hypotension was defined as decrease in systolic blood pressure > 20% from baseline values and or < 90 mmHg immediately after spinal anaesthesia. Patients received increments 6mg mephentermine as required for hypotension. Patients randomly received one of the two study anti-emetic drug combination therapy according to a closed sealed opaque envelope technique: Group Ond + Dexa [n=30] [Dexamethasone (8mg) + Ondansetron (4mg)]: Intravenous Dexamethasone 8mg (2ml) was given immediately before Spinal anaesthesia and Intravenous Ondansetron 4 mg (2ml) was given 20 minutes before completion of surgery. Group Ram + Dexa [n=30] [Dexamethasone (8mg)+Ramosetron (0.3mg)]: Intravenous Dexamethasone 8mg (2ml) was given immediately before Spinal anaesthesia and Intravenous Ramosetron 0.3 mg (2ml) was given 20 minutes before completion of surgery. A specially designed proforma was used to collect the data including patient’s particulars, patient’s written informed consent, indication for surgery, the anesthetic details, intra-operative monitoring, post-operative follow up and PONV scoring system [15]. Thus there is no uniform and consistent scoring system to assess PONV. As the scoring system employed by Kushwaha, et al16 was simple and easy to follow, so Kushwaha, et al16 scoring system of PONV was used. Inj.Diclofenac75mg IM was administered 8th hourly for post operative pain relief. The incidence of nausea, vomiting and retching was studied for a period of 24 hours post operatively. All patients were assessed every hourly for the first 6 Hours, 3 hourly for the next 6 hours and 6th hourly for subsequent 12 hours using the following: PONV scoring system. [16] Score 0: No Nausea Score 1: Nausea only Score 2: Nausea with Retching Score 3: Vomiting For the purpose of this study: Nausea is defined as a subjectively unpleasant sensation associated with an urge to vomit.7 Retching is defined as spasmodic, rhythmic contraction of respiratory muscle without expulsion of gastric contents.7 Vomiting is defined as forceful expulsion of gastric contents.7 Nausea and vomiting occurring within first 6 hours is considered as early nausea and vomiting.7 Vomiting and retching episodes separated by less than 5 minutes as taken as Single episode. Complete response is defined as absence of nausea, retching, vomiting and no requirement of rescue anti-emetic. Patients received Inj Metoclopramide 10mg I V as rescue anti emetic. Patients were also monitored for adverse effects like headache, dizziness, arrhythmia, drowsiness, flushing and sedation in the 24 hour post operative Statistical Analyses: The data was expressed as mean and standard deviation. The homogenicity in two groups of mean and standard deviation was analysed using SPSS version. Comparison between two groups at a time (inter-group comparison) was done using student’s unpaired t- test. P value < 0.05 was considered statistically significant, P value < 0.01 was considered highly significant, P value > 0.05 was considered insignificant. Statistical Software: The statistical software namely SPSS 15.0, Stata 8.0,MedCalc 9.0.1 and Systat 11.0 were used for the analysis of the data and Microsoft word and Excel have used to generate graphs, tables etc. RESULTS The study was conducted to evaluate the efficacy of two prophylactic antiemetic combination therapies with two groups of thirty patients in each of ASA class I and II, scheduled to undergo Elective Total Abdominal Hysterectomy under Spinal Anaesthesia. Patients were randomly allocated to the following two groups
All patients assessed every hourly for the first 6 hours, 3 hourly for the next 6 hours and 6th hourly for subsequent 12 hours using the following PONV scoring system. Score 0= no nausea; 1=nausea only; 2=nausea with retching; 3=vomiting. The results obtained were analyzed after completion of the study. The groups were comparable with respect to age, weight and sex. There was no statistically significant difference observed between groups.
Table 1: Demographic Data
The groups were comparable with respect to age, weight and sex. There was no statistically significant difference observed between groups.
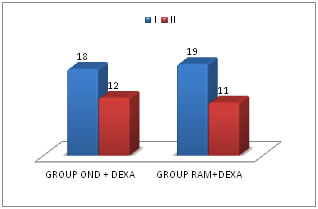
Table 2: ASA Grading of patients studied
Figure 1: Chart showing ASA GRADING
There was no statistically significant difference in the ASA GRADING in all the two study groups. COMPARISION OF PONV SCORES
SCORING SYSTEM USED: Score 0: No Nausea Score 1: Nausea only Score 2: Nausea with Retching Score 3: Vomiting COMBINING THE OBSERVATIONS IN 4- 6 HRS:
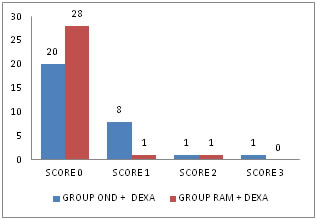
Table 3: Comparison of PONV SCORE IN FIRST 4-6 HOURS
Figure 2: Chart showing PONV in 4-6 hours INFERENCE: Complete response was noted in 66% and 93% in Group Ond + Dexa and Group Ram + Dexa respectively. Incidence of Nausea (Score-1) was 26% and 3% in Group Ond + Dexa and Group Ram+Dexa respectively. Incidence of Nausea with Retching (Score-2) was 3% and 3% in Group Ond + Dexa and Group Ram+ Dexa respectively. Incidence of Vomiting (Score-3) was 3% in Group Ond + Dexa but absent in Group Ram + Dexa in 4 to 6 hours respectively. There was significant clincial and statistical difference P<0.05 noticed between the two groups in 4 to 6 hours. There were no adverse effects noted in any of the groups. COMPARISON OF PONV SCORES BETWEEN TWO GROUPS IN FIRST 3HRS, 4-6HRS, 6-9HRS, 9-12HRS, 12-18HRS and 18-24HRS.
Table 4: Ponv scores in group ond+ dexa in 24 hours
The above table shows the incidence of PONV observed in Group Ond + Dexa in 24hrs. The complete response (Score-0) was 86%, 66%, 93%, 93%, 93% and 93% in first 3 hours, 4-6hrs, 6-9hrs, 9-12hrs, 12-18hrs and 18- 24hrs respectively. PONV Score of Nausea 1 was seen in 3 patients in the first 3hr, 8 Patients in 4-6hrs, Not observed in 6-9 hrs, 1 patient each in 9-12hrs, 12-18hrs and 18 -24hrs respectively. Therefore, the incidence of nausea (score 1) was 10% in first 3hrs, 26% in 4-6hrs,0% in 6-9hrs,3% in 9-12hrs, 3%in 12-18hrs and 3% in 18-24hrs. Nausea with Retching (Score 2) was seen in 1 patient each in first 3hrs, 4-6hrs, 6-9hrs, 9-12hrs and 18 -24hrs respectively. Not seen any patient in 12-18hrs. Vomiting (Score of 3) in 1 patient each in, 4-6hrs, 6-9 hrs and 12-18hrs respectively. In the period of 24 hours, 13 patients did not experience nausea, retching or vomiting and their scores were 0 throughout the study. Therefore, complete response was 43%.Incidence of PONV was 57%. In group Ond+ Dexa, overall incidence of nausea was 53%, retching 16% and vomiting was 3%.
Table 5: Ponv scores in group ram +dexa in 24 hours:
The above table shows the incidence of PONV in Group Ram + Dexa in 24hrs. Majority of the patients had complete response during the study period. Complete response (Score-0) was 96% in first 3 hrs, 93% in 4- 6hrs, 100% in 6-9hrs, 96% in 9-12hrs, 100% in 12-18hrs and 96% in 18-24hrs. The incidence of nausea (score 1) was 3% in 1st 3hrs, 3% in 4-6 hours, 0% in 6-9hrs, 3% in 9-12hrs, 0% in 12-18hrs and 3% in 18-24hrs. Nausea with retching (score 2) was seen in 1 patient in 4-6hrs period. Vomiting (score 3) was not observed in 24 hour study period. In 24 hour period, 25 patients did not experience nausea, retching or vomiting and their scores were 0 throughout the study. Therefore, complete response was 83%. Five patients experienced nausea during the study period. Therefore the incidence of PONV was 17% in Group Ram+Dexa. In Group Ram + Dexa, overall incidence of nausea was 16%, retching 3% and vomiting was 0%. Rescue Antiemetic
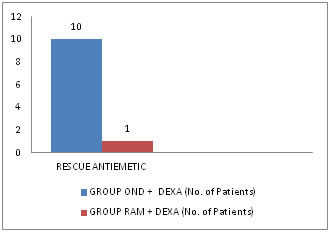
Table 6: Comparison of need for Rescue Antiemetic between the two Groups
Figure 3: Chart showing No. of patients required Rescue Antiemetic There was requirement of rescue antiemetics in 10 patients in Group Ond + Dexa and in 1 patient in Group Ram + Dexa. So the incidence of rescue antiemetic was 33% and 3% in Group Ond+ Dexa and Group Ram+ Dexa respectively. The P value <0.05 was significant.
Table 7: Comparison of adverse effects between groups:
The adverse effects were not observed in the two groups during the study period
DISCUSSION One of the known risk factors for PONV is the female sex, as documented by Apfel, et al.33 In addition laparoscopic surgeries like ovum retrieval and similar gynaecological procedures increase the risk of PONV. Female patients undergoing total abdominal hysterectomy with or without oopherectomy have been considered as a high risk group for PONV and the incidence of PONV in this group has been studied by M.F. Watcha and P.F.White.7 The efficacy of any mono or combination antiemetic therapy is better evaluated in such high risk groups. Hence we chose female patients in the age group between 30-60 years with ASA class I and II, posted for Gynaecological surgeries under spinal anaesthesia. PONV occurs frequently in women undergoing major gyneacological surgery when no prophylactic antiemetic is given.7 This problem is multifactorial in origin and includes age, obesity, a history of motion sickness and previous PONV, menstrual cycle, surgical procedure, anaesthetic technique, and postoperative pain. In the present study major gynaecological surgery i.e. total abdominal hysterectomy cases were selected for two groups as PONV is high in intra-abdominal, pelvic surgeries in female patients. Of the various modes of anaesthetic techniques available, general anaesthesia is found to have the highest incidence of PONV. However, the incidence of intraoperative nausea and vomiting under central neuraxial blockade is reported to be as high as 18% and postoperative vomiting is 21%. The etiology of PONV under spinal anaesthesia may be.
Though spinal anaesthesia is associated with lower incidence of PONV than general anaesthesia, PONV occurrence in gynaecological patients seems to be more dependent on risk factors such as female gender, non smoking status, use of opioids, history of motion sickness or PONV and type of surgery. Most of the gynaecological surgeries are done in our institution under central neuraxial blockade with local anaesthetic-opioid combination intrathecally. Fentanyl is highly lipophilic opioid, which is commonly being used in our institution as an intrathecal additive to local anaesthetics in spinal anaesthesia for its excellent intraoperative and post operative analgesia with better safety profile.17 Hence, we chose this type of anaesthesia in these high risk group patients to evaluate the efficacy of antiemetics. Selection of antiemetic drugs: 5HT3 receptor antagonists: Of the several drugs available to prevent or treat. PONV serotonin (5HT3) antagonists were found to possess significant antiemetic. Activity and effective in Chemotherapy-induced nausea and vomiting (CINV). 5HT3 antagonists were introduced in 1990. The 5HT3 receptor antagonists suppress nausea and vomiting by inhibiting serotonin binding to the 5HT3 receptors present in several critical sites involved in emesis, including vagal afferents, the solitary tract nucleus (STN), and the area postrema. The highest concentration of 5HT3 receptors in the central nervous system (CNS) are found in the STN and chemoreceptor trigger zone (CTZ), and 5HT3 antagonists suppress nausea and vomiting by acting at these sites. They lack the sedative and dysphoric side effects of Droperidol and extrapyramidal side effects associated with high doses of Metoclopramide.18 The commonly used drug of this class is Ondansetron. Ramosetron is a newer 5HT3 antagonist found to be more potent and with a longer half life. Hence we chose to evaluate the efficacy of Ramosetron vs Ondansetron for prevention of PONV in gynaecological surgeries under spinal anaesthesia. Combination of antiemetic: None of the available antiemetics is entirely effective for preventing PONV, especially in high-risk patients. Since at least four major receptor systems are involved in the aetiology of PONV, a better prophylaxis might be achieved by using a combination of agents acting at different receptor sites.12 For example, if the serotonin receptors have already been blocked, consider adding an anticholinergic, antidopaminergic, or antihistamine. The concept of combination antiemetic therapy was first introduced in chemotherapy induced vomiting.12 The most commonly studied combinations have included a 5-HT3 receptor antagonist with either Droperidol or Dexamethasone. Both combination regimens appear to be equally efficacious.12 When Dexamethasone was given concomitantly with a 5HT3 receptor antagonist the absolute risk of PONV was reduced to minimum. Also this combination has minimal adverse effects, most of them due to 5 HT3 receptor antagonist. Hence, the combination of Dexamethasone with a 5HT3 receptor antagonist seems to be a logical choice for the control of PONV.
CONCLUSION
REFERENCES
|
|
 Home
Home