Official Journals By StatPerson Publication
|
Table of Content Volume 11 Issue 2 - August 2019
Serological prevalence of rubella, cytomegalovirus, herpes simplex virus, treponema pallidum and human immunodeficiency virus in antenatal women with bad obstetric history – A cross sectional study
Shama Taj K R
Assistant Professor, Department of Microbiology, Akash Medical College Devanahalli Banglore Rural- 562110 Email: shamatajkr.taj@gmail.com
Abstract Context: Viruses are the most important among the various agents associated with infections of pregnancy followed by bacteria and protozoa. The maternal infections are initially asymptomatic, hence difficult to diagnose on clinical grounds. Infections of Rubella virus (RV), Cytomegalovirus (CMV), Herpes simplex virus (HSV), Treponema pallidum and Human Immunodeficiency virus (HIV) are manageable, hence early diagnosis of them reduces adverse foetal outcome and thus manage Bad Obstetric History (BOH). Aims: The study was intended to observe the serological prevalence of Rubella virus (RV), Cytomegalovirus (CMV), Herpes simplex virus (HSV), Treponema pallidum and Human Immunodeficiency virus (HIV) in antenatal women with BOH. Settings and Design: The present study was a type of cross sectional study conducted at the Department of Microbiology, J.J.M Medical College and SSIMS and RC, Davanagere, Karnataka, over a period of 1 year with a total of 100 antenatal women with bad obstetric history presenting to Chigateri General Hospital and Bapuji Hospital attached to J.J.M. Medical College and SSIMSandRC, Davanagere. Methods and Material: The patients were tested for RV, CMV, HSV, Treponema pallidum and HIV infection in antenatal women with BOH. All the samples were screened by line immuno assay (LIA) using recomLine TORCH Screening IgG/IgM kits used for detection of IgM and IgG antibodies to RV, CMV and HSV agents. Carbogen test for syphilis and Comb Aids RS Advantage HIV 1+2 Immunodot Test, PAREEKSHAK® HIV- 1/2 TRILINE CARD TEST, AIDSCAN® HIV-1/2 TRISPOT TEST for HIV. Statistical analysis Used: The statistical tests used were rate, proportion, nonparametric test. Chi-square test was applied for testing hypothesis regarding qualitative variable. Results: Out of the 100 antenatal women with BOH in the study group, 96% were positive for TORCH infections. Of the total TORCH positive cases 82.29% were positive for IgG alone and 6.25% were positive for both IgG and IgM. Of all the TORCH positive cases, RV accounted for 14.58% of cases, CMV accounted for 23.95% of cases and HSV accounted for 14.58%. Out of 100 cases, CMV accounted for 27.27% followed by HSV (22.72%) and RV (11.36%) in abortions; RV accounted for 15.38%, followed by CMV (15.38%), HSV (7.69%) in congenital anomalies; CMV accounted for 40%, followed by RV (20%) in neonatal death; RV accounted for 20%, followed by CMV (13.33%) in intrauterine death. The combination of RV and CMV was seen in 20% cases of neonatal death and 6.6% cases of intrauterine death. In this study, all the BOH cases were negative for syphilis and HIV positive cases accounted for 2% of the total BOH cases. Conclusions: We conclude that TORCH infections during pregnancy are a major cause of BOH. All antenatal cases with BOH should be screened for TORCH agents as well as Rubella and HIV, as early diagnosis and appropriate management of infections during the present pregnancy will reduce the adverse foetal outcome. Key Word: Antenatal women, bad obstetric history, HIV, LIA, syphilis, TORCH.
INTRODUCTION Bad obstetric history (BOH) implies previous unfavourable foetal outcome in terms of two or more consecutive spontaneous abortions, history of intrauterine foetal death, intrauterine growth retardation, stillbirth, early neonatal death and/or congenital anomalies.1 Cause of BOH may be genetic, hormonal, abnormal maternal immune response and maternal infection. The prevalence of infections caused by Rubella virus (RV), Cytomegalovirus (CMV), Herpes simplex virus (HSV), Syphilis and Human Immunodeficiency virus (HIV) during pregnancy varies from one geographical area to another.2 Most of these TORCH infections cause mild maternal morbidity but have serious foetal consequences and treatment of maternal infection frequently has no beneficial effect for the foetus. They are a group of viral, bacterial, and protozoan infections that gain access to the foetal bloodstream transplacentally via the chorionic villi. These maternal infections with adverse outcome are initially unapparent or asymptomatic and are thus difficult to diagnose on clinical grounds. In cases with previous history of pregnancy wastage, the serological reactions for TORCH infections during current pregnancy must be considered while managing BOH cases to reduce the adverse foetal outcome. Therefore, diagnosis of TORCH infection in pregnant women is usually established by demonstration of seroconversion in paired sera or by demonstration of antibodies.3 The TORCH test belongs to a category of blood tests called infectious disease antibody titre tests which measure the presence of antibodies against a specific group of infectious diseases and their level of concentration in the blood. A positive IgG antibody test is usually a sign of past-exposure to the TORCH agent and is not a marker for current active infection.4 RV is the causative agent of the disease commonly known as German measles. Rubella infection of women during the first trimester of pregnancy can induce a spectrum of congenital defects in the new-born, known as congenital rubella syndrome (CRS).5,6 Congenital infection is due to transplacental transmission and RV generally establishes a chronic non-lytic infection in the foetus and has the potential to infect any organ. WHO estimates that worldwide more than 100,000 children are born with CRS each year, most of them in developing countries. The diagnosis of rubella is very often missed as the infection is mild and the rash and lymphadenopathy are transient. Primary virus infection during pregnancy may lead to teratogenic effects on the foetus. It is highest in cases of infection during first 3 months of pregnancy (40-60%) and progressively decreases during the fourth and fifth months (10-20%).6 To accurately confirm a recent rubella infection, which is critically important in a pregnant woman, either a rise in antibody titre must be demonstrated between two serum samples taken at least 10 days apart or rubella specific IgM antibodies must be detected in a single specimen using ELISA. Following well-designed and implemented programs, Rubella and CRS have almost disappeared from many countries.7 Human cytomegalovirus is a species of the virus genus Cytomegalovirus, which in turn is a member of the viral family known as Herpesviridae.8 CMV is the most common congenital infection and its incidence has been estimated to be between 0.2 - 2.2% of all live births in different parts of the world. CMV infection is typically unnoticed in healthy people, but can be life-threatening for the immunocompromised individuals and new born infants. Global prevalence of CMV infection is reported approximately 40% -80%, but it has been estimated to vary from about 45% in developed countries and to 100% in developing countries.9 CMV infection during pregnancy is far more complex than other infections, due to the ability of the virus to be frequently reactivated during the child bearing age and be transmitted to the foetus in spite of maternal immunity.10 Primary CMV infection occurs in 0.15 to 2.0% of all pregnancies and may be transmitted to the foetus in up to 40% of cases. Up to 15% of intrauterine CMV infections result in symptomatic congenital disease at birth, and 10 to 15% of those born with asymptomatic congenital CMV will develop significant clinical sequelae in infancy. Diagnosis of CMV can be done by human fibroblast culture from urine, saliva or other body fluids. Serological assay can detect CMV IgG antibody indicative of past infection. Detection of viral IgM antibodies suggests current infection. Development of an effective vaccine for prevention of CMV infection would be of great importance. Currently no CMV vaccine is available; it is still in research and development stage. Herpes simplex virus (HSV) is a ubiquitous, enveloped, and double stranded DNA virus, belonging to the family of Herpesviridae transmitted across mucosal membranes and non-intact skin, that migrate to nerve tissues, where they persist in a latent state. Herpes simplex virus type 2 (HSV-2) is the cause of most genital herpes and is almost always sexually transmitted. The acquisition of genital herpes during pregnancy has been associated with spontaneous abortion, intrauterine growth retardation, preterm labour, and congenital and neonatal herpes infections. The risk of neonatal infection varies from 30% to 50% for HSV infections that onset in late pregnancy (last trimester), whereas early pregnancy infection carries a risk of about 1%. When primary HSV infection occurs during late pregnancy, there is not adequate time to develop antibodies needed to suppress viral replication before labour. Syphilis is a multistage disease caused by Treponema pallidum (TP) that is usually transmitted through contact with active lesions of a sexual partner or from an infected pregnant woman to her foetus.11 The World Health Organization estimated that there were 12 million new cases of syphilis in 1999, with more than 90% of the cases occurring in developing countries. Congenital syphilis is a leading cause of stillbirth and perinatal mortality in many of these countries. Congenital Syphilis has been traditionally classified as early congenital syphilis and late congenital syphilis. In early congenital syphilis signs appear in the first 2 years of life while in late congenital syphilis signs appear over the first 2 decades. Syphilis has diverse clinical manifestations and shares many clinical features with other treponemal and non-treponemal diseases. Despite the availability of new diagnostic tests and antibiotic therapy, syphilis has re-emerged in several developed countries.12 HIV/AIDS infection is an important cause of maternal and perinatal morbidity and mortality. Anaemia, pre-term labour, intrauterine growth restriction, foetal deaths, still births and low birth weight are some of the complications associated with HIV in pregnancy.13,14 In pregnancy, immune function is suppressed in both HIV-infected and uninfected women. Vertical transmission from an infected mother to her child is a special problem. Infection can occur in utero or intrapartum. Postpartum infection can result from the ingestion of breast milk by a nursing infant from an infected mother.15 Single-drug treatment or monotherapy against HIV infection is not effective because of the rapid emergence of drug-resistant viral mutants. Multidrug, combination therapy currently is the strategy for HIV management. Primary therapy for HIV infection involves combinations of various antiretroviral drugs. Early antiviral therapy of HIV infection is likely to be beneficial even in asymptomatic subjects. Due to all the above factors, early diagnosis of these infections is essential to start appropriate treatment on time to reduce the transplacental transmission. Most probably due to lack of facilities in isolating etiological agents causing BOH and the prohibitive cost of commercial diagnostic kits, studies analysing the role of maternal infection in the causation of BOH are less in number. In this context, the present study was carried out on antenatal women with BOH. The study was intended to observe the serological prevalence of RV, CMV, HSV, TP and HIV in antenatal women with BOH.
MATERIALS AND METHODS recomLine TORCH Screening IgG kit and recomLine TORCH Screening IgM kit was procured from M/s MIKROGEN Diagnostik, Germany. Carbogen® Rapid Plasma Reagin card test for testing syphilis was procured from M/s Tulip diagnostics, Goa, India. Comb Aids RS Advantage HIV 1+2 Immunodot Test Kit was procured from M/s Span Diagnostics Ltd., Surat, India. PAREEKSHAK® HIV- 1/2 TRILINE CARD TEST, AIDSCAN® HIV-1/2 TRISPOT TEST KIT was procured from M/s Bhat-Biotech India Pvt Ltd., Bangalore, India. Source of data: The present study was a type of cross sectional study conducted at the Department of Microbiology, J.J.M Medical College and SSIMSand RC, Davanagere, Karnataka, during August 2015 and July 2016 with a total of 100 antenatal women with bad obstetric history presenting to Chigateri General Hospital, Bapuji Hospital attached to J.J.M. Medical College and SSIMSandRC, Davanagere. The institutional ethical committee clearance was obtained to conduct the study. Method of collection of data: Blood samples from 100 women with bad obstetric history presenting to Chigateri General Hospital, Bapuji Hospital attached to J.J.M. Medical College and SSIMSandRC, Davanagere were tested for RV, CMV, HSV, TP and HIV infection. A standard case proforma was maintained and study documented under the following important headings;
Note:
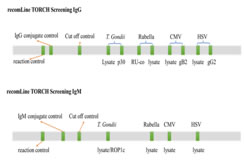
Method of Sample collection: 3ml of whole blood samples from 100 antenatal women with bad obstetric history were collected under aseptic precautions by venipuncture using sterile disposable syringes. Processing of serum samples: After centrifugation, clean serum was transferred into vials and stored at 4-8°C. All the sera samples were tested for RV, CMV, HSV infection by Line immunoassay for IgG and IgM antibodies. VDRL reactive cases were confirmed by Carbogen test for syphilis and Comb aids for HIV, if reactive reconfirmed with Triline test and Trispot test (NACO guidelines). TORCH test protocol: All reagents were prepared as per manufacturer’s recommendation and brought to room temperature for at least 30 minutes before beginning the test. The test strips were placed in 2ml of ready to use wash buffer-A. 20μl of undiluted serum samples were pipetted on to the test strip for each incubation mixture. This was incubated for 1hr with gentle shaking. Diluted serum was siphoned from individual well and 2ml of ready for use wash buffer-A was pipetted into every well. It was washed for 5min with gentle shaking and then the wash buffer-A was siphoned off. Washing step was repeated thrice. Then 2ml ready-to-use conjugate solution was added and incubated for 45min with gentle shaking. Washing step was repeated thrice. Substrate reaction 1.5ml of ready-to-use substrate solution was added and incubated for 8min with gentle shaking. The substrate solution was removed and washed thrice briefly with deionisied water. The strips were dried between 2 layers of absorbant paper for 2hrs before analysis. Interpretation of TORCH test results Interpretation of recomLine TORCH Screening IgG/IgM test was done by two-band strategy as described below. (Figure1)
Time duration of past infection of Toxoplasma and CMV, type specificity of HSV, protective immunity of Rubella is indicated by ancillary band. Figure 1: recomLine TORCH Test
recomLine TORCH Screening IgM test Interpretation of this test was done by single band strategy. The test strips contain one band per infectious agent thus enabling the identification of specific IgM class antibodies to Toxoplasma, Rubella, CMV and HSV type 1 and 2. RecomLine TORCH Screening IgG assay is supported by the recomLine TORCH Screening IgM assay; hence both are done together. Recombinant antigen p30 for Toxoplasma indicates past infection prior to last three months. Recombinant antigen gB2 for CMV indicates past infection prior to last 6-8 weeks. The protective immunity to Rubella is predicted by the presence of higher intensity lysate band compared to Rubella vaccination cut off band. Recombinant antigen gG2 for HSV indicates infections caused by HSV-2. Validation of TORCH test Quality control of the test was assessed by the presence of three bands, namely; Reaction control band, Antibody class band and Cut-off control band. The same procedure was also repeated for IgG antibodies.
Table 1: RV specific interpretations of the test results
Table 2: CMV specific interpretations of the test results
Table 3: HSV specific interpretations of the test results
Carbogen® test: Carbogen test a Rapid Plasma Reagin card test was done for diagnosis of syphilis. Test procedure: 50μl of test specimen, positive control and negative control were pipetted on to separate reaction circles of disposable slide using sample dispensing pipette. One drop of carbogen reagent was added to the test specimen, positive control and negative controls by using reagent dropper provided with the kit. The test specimen and the carbogen reagent were mixed well by using separate mixing stick and allowed to spread uniformly over the entire reaction circle. The slide was continuously rotated on a mechanical rotator at 180 revolutions per minute. Flocculation was observed microscopically at 8 minutes. Result interpretation: The test was considered as reactive when large and medium black floccules against white background, as weakly reactive when small black floccules against white background and as non-reactive when there are no floccules. Combaids – Hiv 1+2 Immunodot Test: All the test reagents were brought to room temperature and the required combs were labelled. The washing buffer was diluted. 0.1ml each of sample and control were added into each micro test well which contain 0.1ml of sample diluents. Comb was placed into the respective well and incubated at room temperature for 10min at room temperature. After incubation, 0.2ml of Colloidal gold signal reagent was added into the micro test wells. Combs were washed for 10min by moving combs forward and backward in wash tray. Combs was placed into micro test wells containing Colloidal gold signal reagent and incubated at room temperature for ten minutes. The washing step was again repeated. The comb was allowed to air dry and the colour development on the spotted area on the tip of the teeth of the comb was noted for reactivity as well as for the control dot appearance. Reference colour index was used to interpret and compare the results. Pareekshak® Hiv- 1/2 Triline Card Test: Kit and samples were brought to the room temperature. Test device removed from the pouch just prior to testing. Device was placed on a flat surface. 10μl of serum or plasma was added into the sample window and allowed to soak in. 20μl of diluents was added into the same sample window. Positive result was read within 10min and Negative result in 20min. Aidscan® Hiv-1/2 Trispot Test Kit Procedure: All the reagents, devices and specimens were brought to room temperature. Then, 20μl of buffer solution was added to the test device. Later, 20μl of either serum or plasma was added followed by addition of 40μl buffer solution. To this mixture, 20μl of gold conjugate was added and again 40μl of buffer solution was added. Results were interpreted as; Negative- when only one red spot (control spot) appeared at the control region "C", indicate that the specimen did not contain antibodies either to HIV -1 or HIV-2 and Positive - a) when two red spots (Control spot and HIV-1 or HIV-2 Spot) appeared at the control region “C” and test region HIV-1 and/or HIV-2 indicate that the specimen was reactive for antibodies to HIV-1 and/or HIV-2. b) when three red spots (Control, HIV-1 and HIV-2 Spot) appeared at the control region "C" and test region HIV-1 and HIV-2 indicate that the specimen was reactive for antibodies to HIV-1 and HIV-2. Statistical methods: The statistical tests used were rate, proportion, nonparametric test. Chi-square test was applied for testing hypothesis regarding qualitative variable.
OBSERVATIONS AND RESULTS In this study of 100 BOH cases, 96 cases showed positive for one or more of the TORCH agents. Seropositivity of TORCH agents is described in Table 4. Table 4: Seropositivity of TORCH agents
Table 5 shows the cases which were positive for IgM, IgG and both IgM, IgG among the total TORCH positive cases. Table 5: Seropositivity of IgM and IgG antibodies among TORCH positive cases
Table 6: shows the seropositivity of IgM, IgG and both IgM, IgG among the various TORCH positive cases.
2 =49.54 Table 7 shows distribution of TORCH infection in various BOH patients with different presentations. In this study CMV was the second most common agent responsible for BOH. Abortion was the commonest mode of presentation of BOH, followed by still birth and IUD.
Table 7: Distribution of TORCH infection in various BOH cases
Out of 12 positive cases of IgG for Rubella, 10 cases were associated with protective immunity and 2 cases were without protective immunity (Table 8).
Table 8: Rubella IgG positive patients with protective immunity
In this current study, out of 21 positive cases of IgG for CMV 17 cases showed past infection of more than 6-8 weeks duration and in the remaining 4 cases the duration was not known (Table 9).
Table 9: Time duration of past infection of cytomegalovirus
Among HSV infection, HSV-2 was responsible for majority of cases accounting for 9 cases out of the 13 cases and the rest were caused by HSV-1 (Table 10).
Table 10: Type specific distribution of herpes infection
In this study, all the BOH cases were negative in Carbogen test done for syphilis and HIV positive cases accounted for 2% of the total BOH cases (Table 11)
Table11: Seropositivity of HIV infections.
DISCUSSION Prenatal screening for antibodies to Toxoplasma, Rubella, CMV, Herpes simplex virus and other agentslike Treponema pallidum, HIV is a routine practice in many parts of the world and is commonly referred to by the acronym TORCH. This screening protocol is most often used to identify pregnant mothers at risk of transmitting viral or protozoan infections in utero to the foetus or to evaluate new-borns presenting with nonspecific, unexplained symptoms thought to be due to infection.16 The TORCH infections are grouped together as all of them result in serious birth defects when transmitted from infected mother to her foetus during pregnancy. TORCH infections lead to mild morbidity in pregnant women but they can cause serious foetal consequences. Since majority of the TORCH infections remain asymptomatic diagnosis of infection in pregnant women is difficult. Demonstration of seroconversion in paired sera or by demonstration of antibodies is required to establish diagnosis of TORCH infection in pregnant women. The present study was conducted at the Department of Microbiology, J.J.M Medical College and SSIMSand RC, Davanagere, Karnataka using recomLine TORCH Screening IgG/IgM kit for TORCH agents, Carbogen® test kit for testing syphilis, Comb Aids RS Advantage HIV 1+2 Immunodot Test Kit for testing HIV and PAREEKSHAK® HIV- 1/2 TRILINE CARD TEST, AIDSCAN® HIV-1/2 TRISPOT TEST KIT for confirmation of HIV. In this study out of 100 cases of BOH cases 96% were positive for TORCH infections. This was similar to a study by Padmavathy M et al in which 98% of the BOH cases were positive for TORCH cases.17 Of the total TORCH positive cases 82.29% were positive for IgG alone and 6.25% were positive for both IgG and IgM. The similar findings were seen in a study by Padmavathy M et al17 in which 85% of cases were positive for IgG alone and 13% were positive for both IgG and IgM. RV accounting for 14.58% of cases, CMV accounting for 23.95% of cases and HSV accounting for 14.58% of all the TORCH positive cases17, this goes in accordance with the study by AL-Taie AA.4 In abortions CMV accounted for 27.27% followed by HSV (22.72%) and RV (11.36%). In congenital anomalies RV accounted for 15.38%, followed by CMV (15.38%), HSV (7.69%). In neonatal death CMV accounted for 40%, followed by RV (20%). In intrauterine death RV accounted for 20%, followed by CMV (13.33%). The combination of RV and CMV was seen in 20% cases of neonatal death and 6.6% cases of intrauterine death. In this study all 100 cases of BOH were negative for syphilis. This was similar to a study by Padmavathy M et al in which all were negative for syphilis.17 Further, in this present study HIV positive cases accounted for 2% of the total BOH cases.
CONCLUSION The conclusions drawn from the study are;1 TORCH infections are associated with BOH and they are an important cause for recurrent abortion, congenital anomalies, birth, neonatal death and intrauterine death.2 The adverse foetal outcome can be reduced by carrying out serological tests for TORCH infections during the current pregnancy in all pregnant women presenting with previous history of pregnancy wastages and appropriate treatment to reduce the transmission of the infection to foetus in cases with positive serological reactions.3 The TORCH infections during pregnancy are known causative factor for causing adverse foetal outcome and resulting in BOH. TORCH infections are treatable, hence early diagnosis and appropriate management reduces adverse foetal outcome in BOH.4 All antenatal cases with BOH even if asymptomatic, they should be routinely screened for TORCH agents as early diagnosis and appropriate management of TORCH infections during the present pregnancy will reduce the adverse foetal outcome.
REFERENCES
|
|
 Home
Home