Official Journals By StatPerson Publication
|
Table of Content Volume 12 Issue 1 - October 2019
Bacteriological study of chronic suppurative otitis media (CSOM) amongst adult patients
Pokharnikar S1, D’Souza D2*, Baveja S3
1Resident Medical Officer, 2Assistant Professor, 3Professor and HOD, Department of Microbiology, 4th floor, College building, LTMMC and GH Sion Mumbai 22, Maharashtra, INDIA. Email: drdesma@gmail.com
Abstract Background: Chronic Suppurative Otitis Media (CSOM) is a common infection encountered in Ear Nose Throat (ENT) practice throughout the world. It is one of the important cause of hearing loss and morbidity. Early and effective treatment based on the knowledge of causative micro-organisms and their antimicrobial sensitivity ensures prompt clinical recovery and possible complications can thus be avoided. Hence the present study was undertaken to identify the common microorganisms isolated in adult patients with CSOM and to study the antimicrobial susceptibility pattern of aerobic isolates. Method: 120 clinically diagnosed adult patients of CSOM attending ENT OPD were included in the study. The relevant clinical details were recorded and ear swabs for bacterial culture were collected from these patients and were analyzed in the Department of Microbiology. Identification of organisms and antimicrobial susceptibility was done for all aerobic and anaerobic isolates using standard bacteriological techniques. Results: Aerobic growth was seen in 87.5% and anaerobic growth in only 3.33% of the specimens. The most common causative organisms isolated were Pseudomonas aeruginosa (50.83%) followed by Staphylococcus aureus (23.33%) among the 110 aerobic isolates. Antimicrobial profile of Pseudomonas aeruginosa showed maximum sensitivity to Imipenem (100%) followed by Piperacillin-tazobactam (98.37%) and Ciprofloxacin (86.8%). Among the Staphylococcus aureus isolates 93.4% were MSSA and only 6.6% were MRSA. Conclusion: Aerobic bacteria were most prevalent in this study. Knowledge of the etiological agent and appropriate and judicious use of antibiotics as per microbiological susceptibility reports will help to reduce development of resistant strains and also decrease rate of complications in long run. Key Words: Chronic Suppurative Otitis Media (CSOM), Aerobic, Pseudomonas aeruginosa, Staphylococcus aureus, Enterobacteriaceae, Imipenem, Piperacillin-Tazobactam.
INTRODUCTION Chronic Suppurative Otitis Media (CSOM) is defined as chronic inflammation of middle ear and mastoid cavity that may present with recurrent ear discharges or otorrhoea through a tympanic perforation 1. It is commonly a disease of developing world and incidence is increasing during past 10-20 years. Risk factors that have been attributed to the high rates of CSOM in these populations are: overcrowding, poor hygiene, poor nutrition, high rates of nasopharyngeal colonization with potentially pathogenic bacteria and inadequate and unavailable health care.2 According to WHO, countries were categorized by CSOM prevalence rate into those having low infection when prevalence rate of ear infection is 1-2% and high when it is 3-6%. India has a prevalence rate of >4% and comes under highest infection category which needs urgent attention to deal with massive public health problem. CSOM was found to be responsible for hearing impairments in 77% of cases in India. India accounts for most deaths (12 per thousand) and years of life lost and DALYs from otitis media according to WHO data 3.The widespread use of antibiotics and antihistaminics has led to changes in the microbial flora and also to emergence of multiple resistant strains. The study of microorganisms commonly associated with CSOM and their antimicrobial susceptibility pattern is very pertinent for the clinician to plan general outline of treatment for patients with chronically discharging ears 4. The aim of this study was to determine the most frequent bacteria that cause chronic suppurative otitis media and their sensitivity to various antimicrobials so that an appropriate therapy can be started promptly while awaiting the results of the culture and sensitivity of the isolate to various antibiotics.
MATERIALS AND METHODS The present observational study was conducted after obtaining the institutional ethics clearance. Patients with clinical evidence of CSOM attending the Outpatient Department of ENT section of tertiary care hospital were included over a period of one year. Total 120 patients of age ≥ 14 years and either gender, having persistent ear discharge of ≥ 6 week’s duration and presence of tympanic membrane perforation, who have not received antibiotic therapy (topical or systemic) for previous 14 days, were enrolled through convenience sampling. Patients with age <14 years, having duration of ear discharge < 6 weeks, ear discharge without tympanic membrane perforation, and patients with any ear surgery within the last six months were excluded from the study. An informed consent was obtained from all the patients. A detailed clinical history regarding age, sex, duration of discharge and other relevant details was recorded. Sample collection and transport: Two thin sterile cotton swabs were used to collect the pus for bacteriological study. One swab was processed for aerobic culture and smear preparation and other swab was inoculated in Stuart‘s transport medium 5 for anaerobic culture. Aerobic culture 6: Pus for aerobic culture was inoculated on Blood agar, Chocolate agar and MacConkey agar plates and a smear was prepared on a clean grease free glass slide. After inoculation Blood agar and MacConkey agar, plates were incubated aerobically at 37°C and Chocolate agar in candle jar for 24-48 hours. Organisms were identified using standard procedures 7 and antimicrobial susceptibility was done for all aerobic isolates by Kirby Bauer disc diffusion method on Mueller-Hinton agar plate according to CLSI 2013 guidelines 8. Anaerobic Culture 9, 10: The swab from Stuarts transport medium was plated on Neomycin Blood Agar (NBA), Wilkins Chalkgren Agar (WCA), Bacteroides Bile Esculin (BBE) agar and also inoculated in Thioglycollate broth. All the plates were incubated in MacInthosh Fildes‘Jar under anaerobic conditions for 48 hrs. Thioglycollate broth was incubated with lid tightly capped at 37 °C for 48 hours. At the end of 48 hrs the plates were examined for any growth. Also, after 48 hours a loop full of Thioglycollate broth was sub cultured on Neomycin Blood Agar, Wilkins Chalkgren Agar Bacteroides Bile Esculin agar and incubated in MacInthosh Fildes‘Jar under anaerobic conditions for 48 hrs. Identification of anaerobic isolates was done as per standard procedures 9-12. Statistical Analysis The data was analyzed by using Statistical Package for Social Sciences (SPSS) version 20 and the prevalence of organisms was determined and expressed in percentage. Chi square test was applied for comparison of different age groups amongst gender.
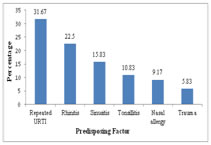
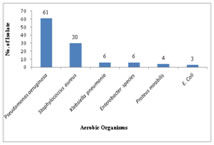
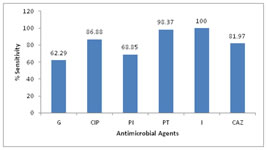
OBSERVATIONS AND RESULTS A total of 120 adult patients of CSOM were included in the study. The maximum numbers of patients belonged to 14-20 years age group, as shown in Table 1. Out of these, 56 (46.67%) were males and 64 (53.33%) were females giving Male: Female ratio of 1: 1.14. 100 (83.33%) patients had unilateral infection, out of which 83.33% had unilateral and 16.67% patients had bilateral CSOM. History of Repeated attacks of upper respiratory tract infections (URTIs)was observed as most common predisposing factor as shown in Figure 1. Ear discharge (100%) was observed as the most common presenting symptoms among the patients studied (Table 2). Out of 37 patients who had hearing loss, 31 patients undergone pure tone audiometry. Among these patients, 17 (54.83%) patients had mild hearing loss, 8 (25.80%) had moderate while 5 (16.13%) patients had severe hearing loss. Of 120 patients with ear discharge, 36.67% patients had mucoid type of discharge, 53.33% had mucopurulent while only 10% patients had purulent type of ear discharge. Out of 120 samples, 88.33% samples showed growth while remaining 11.67% samples did not show any growth in culture (Table 3). Of 110 isolates 80 (72.73%) were gram negative bacilli while 30 (27.27%) were gram positive cocci. Pseudomonas aeruginosa was predominant isolate [61 (50.83%)] followed by Staphylococcus aureus [30 (23.33%)], as shown in figure 2. Among the 4 anaerobic bacteria isolated, 3 were gram positive anaerobic cocci with Peptococcus niger, Ruminococcus productus and Anaerococcus parvulus one each and 1 gram negative bacillus i. e. Fusobacterium species. Thus anaerobes were not found to be significantly associated with CSOM in the present study. In the present study, Pseudomonas aeruginosa showed maximum sensitivity to imipenem (100%) followed by piperacillin-tazobactam combination (98.37%),ciprofloxacin (86.88%) and ceftazidime (81.97%). Least sensitivity was noted with gentamicin (62.29%) as shown in figure 3. Out of 30 isolates of Staphylococcus aureus, 28 were of MSSA and only 2 isolates were found to be MRSA. All MRSA were sensitive to vancomycin, linezolid, netilmycin and cotrimoxazole. Amongst MSSA maximum sensitivity was observed to cefuroxime (100%) followed by clindamycin (96.43%). The other gram negative bacilli isolated belonged to Enterobacteriaceae family. Overall Enterobacteriaceae members were 100% sensitive to both imipenem and piperacillin-tazobactam. Good percentage of sensitivity was observed to gentamicin (84.21%) followed by ciprofloxacin (63.16%) and piperacillin (52.63%). Least sensitivity was observed to cefotaxime (36.84%) and Amoxycillin- clavulinic acid (10.53%).
Table 1: Age Distribution of patients
Table 2: Analysis of presenting symptoms in cases of CSOM
Table 3: Types of growth and growth pattern in aerobic culture
Figure 1 Figure 2 Figure 3 Figure 1: Predisposing factors observed in CSOM patients; Figure 2: Distribution of aerobic organisms isolated (n=110); Figure 3: Antibiotic susceptibility for Pseudomonas aeruginosa G-Gentamicin, Cip-Ciprofloxacin, Caz-ceftazidime, Pi-piperacillin, Pt-Piperacillin-tazobactam, I-Imipenem
DISCUSSION CSOM is a major public health problem in India. The present study included 120 clinically diagnosed adult patients of CSOM and maximum number of patients were in the age group of 14-20 years followed by 21-30 years. The high prevalence of otitis media in in young adults may be attributed to the fact that they are usually more prone to develop upper respiratory tract infections (URTIs). It was observed that with the increasing age there was decrease in the percentage of cases of CSOM. Similar observation was also made by Rajat Prakash et al 13, V. K. Poorey4 and Shrestha B.L.et al 14 who observed maximum patients in both genders belonging to 0-20 years of age. Analysis of the gender incidence revealed that otitis media was found to be more common in females than in males. As this study involved a random selection of cases, the prevalence of male patients over female patients may be only an incidental finding. Similar observation were also made by Shrestha B.L.et al14 and by Rajat Prakash et al 13 who reported M:F ratio of 1:1.2. P.K.Maji 15 et al reported male predominance with M:F ratio of 1.3:1. In the present study, among the predisposing factors studied history of repeated attacks of URTI was observed as most common factor in 31.67% patients. This finding was similar to observations made by V. K. Poorey 4. According to Fliss et al 16 significantly increased risk for CSOM was associated with a history of acute and recurrent otitis media, a parental history of chronic otitis media, larger families and more siblings, a higher crowding index and care in large day care centres. The sex, parental age and education, allergy, sinusitis and recurrent upper respiratory tract infections were not associated with CSOM. In the present study ear discharge(100%) and hearing loss (30.8%) were the major presenting symptoms. similar findings were observed by Nikakhlagh et al 17. However, B.N.Rao[18] and Haider A19 reported pain, itching and hearing loss as common presenting symptoms. Out of 120 samples, 106 samples showed growth while remaining 14 samples did not show any growth in culture. The aerobic organisms were isolated from 105 of the samples and anaerobes from 4 of samples. These findings are comparable with the other studies by Ahmad S et al and Iqbal K et al. 20, 21. Contrary to this R.D.Kulkarni 5 isolated anaerobes in 30% cases and Nikakhlagh 17 in 12%. From 100 samples of aerobic growth single organism was isolated while from 5 samples two organisms were isolated giving total of 110 aerobic isolates. Similar patterns of growth were reported by Haider et al who reported monomicrobial growth in 80.7% and polymicrobial growth in 3.3% cultures. 19. Contrary to our findings Yousuf A. et al22 reported higher rates of polymicrobial growth in 32.8% of cultures. The microbiology of CSOM varies from time to time and place to place. Although the common microorganisms isolated from CSOM cases remain Staphylococcus aureus and Pseudomonas aeruginosa, the percentage of these organisms differs according to different studies by Rakesh Kumar23, V.K.Poorey4, and Mariam et al 24. Organisms like Proteus, Klebsiella, E.coli, and Citrobacter can also be the causative agents of CSOM. The significance of anaerobes in aetiology of CSOM is variable according to different studies. They are mostly detected in cases of cholesteatoma and extensive granulation tissue. In present study, Pseudomonas aeruginosa was predominant isolate followed by Staphylococcus aureus which are in concordance with studies by Shrestha B.L, Kumar R and Iqbal K., ShyamalaR, 14,21, 23, 25. The anaerobic organisms isolated were Peptococcus niger, Ruminococcus productus, Anaerococcus parvulusand Fusobacterium species, which is comparable with the study done by Shrivastava et al 26. Pseudomonas aeruginosa showed maximum sensitivity to imipenem followed by piperacillin-tazobactam combination, ciprofloxacin and ceftazidime. Least sensitivity was noted with gentamicin. This results correlated with the study done by Gul et al 27.Only 2 of 30 Staphylococcus aureus isolates were found to be resistant to cefoxitin (MRSA) while 28 were sensitive to cefoxitin (MSSA). All MRSA were sensitive to vancomycin, linezolid, netilmycin and cotrimoxazole. Amongst MSSA maximum sensitivity was observed to cefuroxime followed by clindamycin, cotrimoxazole, gentamicin and erythromycin. Least sensitivity was seen to penicillin. These finding corroborates well with the observations made by Iqbal K, Arvind N, and Agarwal A.21,28, 29. Overall Enterobacteriaceae members were 100% sensitive to both imipenem and piperacillin-tazobactam. Good percentage of sensitivity was observed to gentamicin (84.21%) followed by ciprofloxacin(63.16%) and piperacillin (52.63%). Least sensitivity was observed to cefotaxime (36.84%) and Amoxycillin- clavulinic acid (10.53%). Arvind et al 28 reported 100% sensitivity to imipenem and 54% to ciprofloxacin among the Enterobacteriaceae.
CONCLUSION Pseudomonas aeruginosa and Staphylococcus aureus were found to be more common isolates and anaerobes were not significantly associated with CSOM in the present study. Empirical therapy can be initiated but culture sensitivity should be advised in all cases and treatment modified as per the reports since the pattern of microbial flora and antimicrobial sensitivity vary in CSOM patients. Appropriate and judicious use of antibiotics as per microbiological susceptibility reports will help to reduce development of resistant strains and also decrease rate of complications in long run. REFERENCES
|
|
 Home
Home