Official Journals By StatPerson Publication
|
Table of Content Volume 12 Issue 2 - November 2019
Comparison of automated and conventional culture in the detection of extrapulmonary tuberculosis
Anu P John1*, Beena Philomina J2
1Assistant Professor, Department of Microbiology, Government Medical College, Thrissur. 2Professor & HOD, Department of Microbiology, Government Medical College, Kozhikode. Email: dranupjohn@gmail.com
Abstract Background: There has been a surge in the number of extrapulmonary tuberculosis cases in the recent years. Isolation of M. tuberculosis from clinical samples by culture is the “gold standard” for a definitive diagnosis of EPTB. Isolation by Mycobacterial culture is difficult especially due of the paucibacillary nature of extrapulmonary TB. Aim: To compare automated and conventional mycobacterial culture in terms of positivity, time taken for detection, contamination rates and overall cost benefit. Setting and Design: This study on extra-pulmonary tuberculosis was conducted in the department of Microbiology, Medical College, Kozhikode for a period of one year. Materials and Methods: All the specimens received during the one year period were included in the study. History, clinical examination findings and results of relevant investigations were obtained and recorded in a proforma. All specimens were processed by modified Petroff’s method and subjected to smear microscopy. The processed specimens were cultured by both MB/Bact and Lowenstein- Jenson medium(LJ medium). Results: Out of the 118 specimens included in the study. Isolation was obtained in 14 sample from MB/Bact culture and in 4 samples by LJ culture Isolation rate by automated method was 11.86% whereas by conventional method it was only 3.38%. Average detection time was 14.7 days by automated method and 34 days by conventional LJ medium culture. The contamination rate by automated method was 5.08% and by conventional method it was 17.79%. On comparing with MB/Bact, the specificity of LJ medium is 92.1% while the sensitivity is only 28.57%. MDR TB was detected in 28.85% patients. Conclusion: Automated mycobacterial culturing system is rapid and less labor-intensive which may be considered as a valuable alternative to the conventional culture especially in cases of smear negative extrapulmonary tuberculosis.
INTRODUCTION Extrapulmonary TB (EPTB) represented 14% of the 6.4 million incident cases that were notified in 2017, ranging from 8% in the WHO Western Pacific Region to 24% in the WHO Eastern Mediterranean Region1. The annual incidence of EPTB has been increasing due to the changing TB control practices, the population growth and the cure of infectious cases of TB have resulted in a relative rise of EPTB case detection. HIV further complicates the situation, as EPTB constitutes more than 50 % of all cases of TB in HIV-positive patients2. In TB-endemic countries, TB control programmes focus on management of sputum smear-positive tuberculosis in an attempt to control the epidemic and extra pulmonary tuberculosis receives little public health emphasis as it tends to be paucibacillary. Early diagnosis of tuberculosis and initiating optimal treatment would not only enable a cure of an individual patient but will also curb the transmission of infection and disease to others in the community. Although acid fast bacilli microscopy, and conventional Lowenstein- Jensen medium culture is considered as the cornerstone of the diagnosis of tuberculosis, these traditional bacteriological methods are either slow and their sensitivity is low in samples contain small number of organism. This has necessitated the use of quicker and accurate methods like automated mycobacterial culture3. The main disadvantage of culture is that its results take between 2 and 6 weeks to be ready in solid culture media.. As to minimize this period, liquid culture mediums have been developed and they enable the detection of bacterial growth between 7 and 10 days before solid media4.
MATERIALS AND METHODS This study on extra-pulmonary tuberculosis was conducted in the department of Microbiology, Medical College, Kozhikode for a period of one year. The study was approved by our Institutional Ethics Committee A total of 118 specimens were included in the study. History, clinical examination findings and results of relevant investigations were obtained and recorded in a proforma. All specimens were collected under universal aseptic precaution in suitable sterile containers. In case of any delay, sample was refrigerated and processed within 24 hours. All specimens were processed in Biosafety cabinet class II. Direct smears prepared from of all specimens and Ziehl-Neelsen staining was done. Processing was done by modified Petroff’s method5. In the case of sterile fluids, the specimen was transferred into a 50 ml sterile conical bottom graduated centrifuge tubes. Equal volume of 4% NAOH was added, Vortexed for 15-30 seconds and incubated at room temperature for 10 minutes. The samples were agitated during incubation for better liquefaction. Distilled water was added up to 45ml mark of centrifuge tube. The tube was closed tightly. It was mixed by just inverting the tube and centrifuged at 3000g for 20 minutes. The supernatant was decanted and sediment re suspended with 1ml of distilled water. For biopsy and bone marrow specimen they were added aseptically into sterile mortar. The specimen was ground with minimal volume of distilled water by using sterile pestle, and then decontaminated using Modified Petroff’s method. Urine samples were first centrifuged for 15 minutes at 3000g .The supernatant was decanted and proceeded like body fluids. CSF being from sterile site, was not decontaminated. The processed samples were simultaneously inoculated into MB/Bact bottle and two slopes of LJ medium. Each MB/Bact bottle was labeled with appropriate patient information. The bottles were equilibrated to room temperature before inoculation. Septum was disinfected with alcohol and allowed to dry.0.5ml of reconstituted MB/BacT antibiotic supplement (MAS) was added to culture bottle for non sterile specimens and for sterile specimens 0.5 ml of MB/BacT Reconstitution fluid was added as such. Then 0.5ml of the processed sample was added. Inoculation was done through the rubber septum by means of a syringe. The inoculated bottles were loaded into MB/BacT instrument. The processed samples which were inoculated into two slopes of LJ medium are incubated at 37ºC. BacT/ALERT MP bottles which flagged positive by the instrument were unloaded. The large clumps were broken by vortexing and were suspended uniformly. A small portion of broth was draw from positive bottle by using sterile syringe and needle. Two smears were made, one for acid fast staining and one for gram stain. Growth of Acid fast bacilli was confirmed by Ziehl-Neelsen staining. Subculture was done on LJ medium and blood agar from positive bottles. BacT/ALERT MP Bottles were declared negative for AFB after 42 days of incubation and the corresponding LJ slopes after 12 weeks of incubation5. MTB was differentiated from Non-tuberculosis mycobacterium by the heat stable catalase test. 0.5 ml of growth emulsion from LJ slope in phosphate buffer was heated in water bath at 68ºC for 20 minutes. To this 0.5 ml of freshly prepared Tween peroxide mixture was added and looked for the development of effervescence6. Antibiotic sensitivity of all the isolates to first line drugs were done by proportion method using automated system. The broth culture from positive bottles was adjusted to McFarlands standard 1 by adding distilled water.1/100 dilution of this standard was also made. For doing sensitivity 6 BacT/ALERT MP bottles are required for doing sensitivity. 0.5 ml of restoring fluid was added to all 6 processing bottles, 0.5ml of reconstituted antibiotics, INH, Rifampicin, Ethambutol and Streptomycin were added to four of the bottles. To the rest of the two bottles 0.5ml of distilled water was added. 0.5ml of undiluted broth culture was inoculated into the bottles containing antibiotics and to a fifth bottle which is taken as direct control. 1/100 dilution broth was added to the sixth bottle which is the proportional control7. Bottles were loaded into the instrument. The flagged bottles were subjected to Ziehl-Neelsen staining. The antibiotic incorporated in the bottle which was flagged after proportionate control was considered as susceptible. Resistance was reported if the antibiotic bottle flagged positive before or along with proportionate control bottle. Direct control broth becomes positive earlier than other bottles. Drug susceptibility of 10 isolates to isoniazid (INH), rifampicin (RIF), and ethambutol (EMB) was performed by proportion method on LJ medium8. The concentrations used were 0.2, 2and 40μg/ml for INH, Ethambutol and Rifampicin respectively. The growth from a 3-4 week old culture was scraped with a loop and bacterial suspension was made in sterile distilled water, vortexed and matched with McFarland tube No.1. It was then diluted 1:10 in phosphate buffer saline. The diluted sample was inoculated on both the control and drug containing media and incubated at 370c. The first reading was taken after 28 days of incubation and the second reading on 40th day. The percentage resistance (R) was calculated as the ratio of the number of colonies on the drug containing media to those on the control medium. R (%) = No. of colonies on drug media × 100 No. of colonies on control medium If R = >1 per cent, the isolate was taken as resistant.
RESULTS A total of 118 samples from suspected extrapulmonary tuberculosis were received in microbiology lab for culture The present study demonstrated that automated method provided better isolation rate of mycobacterium (11.86%) than LJ media (3.38%). Culture positivity within 20 days was 71.42% by automated method while it was only 25% by conventional culture. [Table 1].
Table 1: Comparison of time of appearance of growth by automated and conventional methods
Average detection time was 14.7 days by automated method and 34 days by conventional LJ medium culture. Average detection time was 12.2 and 32 days respectively by automated and conventional culture in the isolates from smear positive samples where as it was 17.7 and 36 days in the isolates from smear negative samples. Minimum detection time for MB/Bact system was 6days and for LJ medium it was 18 days. The contamination rate by automated method was 5.08% and by conventional method it was 17.79%.[Table 2].
Table 2: Comparison of detection time of the isolates by the two culture systems
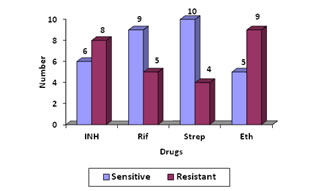
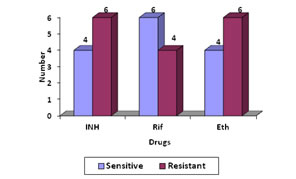
On comparing with MB/Bact, the specificity of LJ medium is 92.1% while the sensitivity is only 28.57%. Among the 118 patient under study, 20(16.94%) patients were on ATT. Of these 20 patients, isolation was obtained in 7(35%) cases. Drug suseptibilty of the 14 isolates was done by both automated and conventional methods .Four strains failed to grow on LJ medium. Of 14 isolates 3 (21.42%) were pan sensitive strains and 2 (14.28%) were pan resistant by automated method. Monoresistance to ethambutol was seen in 2 strains. [Figure 1]. Average detection time for resistance Figure 1 Figure 2 Figure 1: Drug susceptibility pattern of the 14 isolates by automated method; Figure 2: Drug susceptibility pattern of 10 isolates by conventional method Figure 1, Average detection time for resistance by automated method was 7.2 days. Sensitivity of the ten isolates done by LJ medium, 2 were pan sensitive strains and 2 were pan resistant strains. Monoresistance to Ethambutol and Rifampicin was seen in one strain each. Figure 2, Except for two isolates, all others showed the same pattern of sensitivity by both the methods. Out of the 4 MDR strains, primary drug resistance was seen in 1 and acquired drug resistance in 3 strains. Out of the 3 acquired drug resistant cases 2 were defaulters and 1was treatment failure. DISCUSSION The present study demonstrated that automated method provided better isolation rate of mycobacterium (11.86%) than LJ media (3.38%). This means the isolation rate by MB/Bact was 28.49% more than that by LJ method. In the present study the sensitivity of automated culture was far superior to those of the conventional methods (P<0.0001). In a study conducted by Oberoi and Kaur H9 in 2002 showed that rate of isolation by automated method was 14.55% and by conventional method is 2.2%. In another study conducted by Dnyaneshwari P. Ghadage and Archana B. 20/21(95.23%) were detected positive by MB/Bact while 6/21(9.09 %) were positive on solid culture10. Therefore, we believe the low yields of culture by both conventional and automated methods was likely due to the paucibacillary load in the specimens, which was further compounded by use of a small sample volume or biopsy material. Thus, the performance could perhaps be improved further by processing larger volumes/amounts of specimen. It is seen that comparative studies of this two method has certain limitation. First, the study design could account for the decreased sensitivity of the LJ slants since each slant received approximately 0.1 ml of specimen versus the 0.5ml inoculated into MB/BacT and second, the reading frequency was not the same for both media. For automated, reading was done daily and for LJ media once weekly. The mean time of detection in the present study was 14.7 days for automated and 34 days for conventional culture. In a comparative study conducted by Homorodean D11 the mean time for detection by MB/Bact was 14.56days and by L J medium it was 28.66 days. The mean isolation time was 16 days by MB/Bact method, and 26 days by conventional method in the study conducted by Obroi et al9. The mean detection time of mycobacteria by the colorimetric and conventional method in the study conducted by B.Mahadev et al 12 were 14 and 24 days respectively. In thi s study the mean turnaround time for detection of MTB in smear-positive specimens was 12.2 days (range, 6–14 days) by automated method and 32 days(range,18-42) by conventional method. The turnaround time for detection of MTB in smear-negative specimens was 17.7 days (range, 8–38 days) by automated culture and 36 days by conventional method. Minimum time for detection of AFB was 6 days for MB/BacT and 18 days for L J in the present study. A study by B. Mahadev et al12 has shown 5 days as minimum detection time by MB/Bact and for LJ medium it was 12 days. In a study conducted by Obroi et al9 the minimum detection time was as short as 14 hours by colorimetric method and 11 days by LJ method. According to the Center for Disease Control and Prevention recommendations for mycobacteriology performance clearly states that reports of isolation and identification of M. tuberculosis complex species should be available within 21 days of specimen collection13.The contamination rate in this study was 5.08% by automated culture and 17.79% by conventional culture. 6 specimens proved to contaminate both systems with additional 16 (12.7%) specimens contaminating L-J media alone. Contamination must have originated during specimen collection. Additional (12.7%) contaminations of L-J media might have occurred due to subsequent handling of the LJ medium. The contamination rate of 5.08% by automated culture fulfils the CDC requirement13. The accepted contamination as per CDC requirement is 5%. In the study conducted by Oberoi and Kaur9 rate of contamination by MB/BacT was 14.4% and 24.4% in LJ medium which was higher than present study. The break through contamination rates in study conducted by S.rishi et al 14 was13.4% for automated and 27.2% for L. The high rate of contamination may be attributed firstly to the hot climatic conditions of our country and secondly to the longer transporting time of the specimen to the laboratory in some cases, leading to overgrowth of contaminants. During the evaluation of the MB/BacT system, we appreciated the closed vials and their automated reading during the entire incubation period, which required no further handling once the samples had been inoculated and the vials had been registered in the instrument. The contamination rate of LJ was much higher when compared to MB/BacT. Grossly contaminated with LJ medium slopes were discarded while samples that grew both contaminants and mycobacterium in MB/BacT could be considered. The lack of antibiotics in the LJ medium may probably be a reason for its higher contamination rates. Of the total patients twenty patients received ATT. Of the 20 patients, isolation was obtained in 7 cases. In the 7 culture positives, two patients were started on ATT with clinical suspicion before sending samples for culture. 5 patients were already on ATT for pulmonary tuberculosis. Of the 14 culture positives, those 7 patients who were not on treatment were put on ATT after sending the samples for culture. Of the total 20 patients on ATT, five patients received anti tuberculous treatment for a duration of six to nine months and 11 patients were treated for nine to 12 months. One patient was treated for more than one year. Three patients had not completed the full course of treatment. Two patients suffered from polyneuropathy, three had liver enzyme abnormalities. All the 14 isolates were tested for drug susceptibility to streptomycin, isoniazid, rifampicin and ethambutol by the proportion method in MB/BacT (automated method).In the present study, 3 (17.64%) were pan sensitive strains and 2 (11.76%) were pan resistant. One strain (5.88%) showed resistance to single drug ethambutol. 5 strains were resistant to 2 drugs. (29.41%).Out of this one strain each was resistant to INH and rifampicin, rifampicin and ethambutol, INH and Streptomycin. Two strains were resistant to INH and ethambutol. (11.76%). Resistance to three drugs were seen in 2 strains (11.76%). One strain showed resistance to INH, rifampicin and ethambutol and the other strain to INH, streptomycin and ethambutol. In a study by Shah AR in 200215, 58.64% were resistant to one or more drugs. Resistance to one drug was observed in 10.46%, Single drug resistance was most commonly seen with INH 7.5%, followed by streptomycin 1.4%, rifampicin 0.97% and ethambutol 0.4% .Strains resistant to two drugs were 18.13%, to three drugs 14.84% and to four drugs 15.21%. 10 isolates were tested for drug susceptibility to isoniazid, rifampicin and ethambutol by the proportion method on LJ medium. 4 cultures from CSF specimens failed to grow on LJ medium. This may be due to the low numbers of organism present in the culture and more over LJ is less sensitive than MB/bacT. Of the ten, 2 were pan sensitive strains and 2 were pan resistant. Monoresistance to ethambutol and rifampicin was seen in one strain each. 4 (25%) strains were resistant to two drugs. Out of these 4 strains, 3 were resistant to INH and ethambutol and 1 to rifampicin and INH. In a study by Isa et al16 (57.5%) isolates were sensitive to all four first-line drugs. 20% were pan resistant and 16% resistant to INH and rifampicin. 13% were resistant to INH, rifampicin and ethambutol and 16% to INH, rifampicin and streptomycin. The sensitivity of 10 strains isolated by automated method was compared with the conventional method. The isoniazid sensitivity results agreed for 9 of 10 isolates (90% agreement). The rifampicin results agreed for the 10 strains tested (100% agreement). The ethambutol results agreed for 9 of 10 strains (90%). Our data suggest the excellent ability of the MB/BACT system to detect true resistance and true susceptibility against isoniazid and rifampicin, the two major front-line antituberculous drugs. Among the first-line antituberculous drugs, ethambutol very often yields less reproducible results. A quality assurance program for drug susceptibility testing of M. tuberculosis was initiated in 1994 by the World Health Organization in 16 laboratories around the world. The first round of proficiency reported in 1997 yielded lower sensitivity values for ethambutol than for isoniazid and rifampin (66%, 99%, and 94%, respectively). In the second round of proficiency reported in 2002, the sensitivity of testing of ethambutol was less reliable, although it increased from 60% in round 1 to 98% in round 517. In this study mean time for obtaining SIRE susceptibility results was 7.2 days . In another study conducted by Bemer et al18 the mean time was 6.6 days. In a study by Mahadev et al12 mean time was 13.7days. The susceptibility test results with the MB/BacT system was obtained in 7 days in a study by María S.Díaz- Infantes et al19 in Spain. Centres for Disease Control and Prevention recommendation that susceptibility test results for Mycobacterium tuberculosis complex isolates should be available, on an average, 28 to 30 days from the receipt of specimen in the laboratory13.In the present study there were 4 (28.85%) MDR strains. MDR strains are those which are resistant to both INH and rifampicin. Of the 4 MDR strains, primary drug resistance was seen in 1 and acquired drug resistance in 3 strains. Out of the 3 acquired drug resistant cases 2 were defaulters and 1was treatment failure. In a study by RB Deoskar et al20 MDR strains were found to be 6%. In a study by Ravindran et al21 MDR strains were present in 8.8%. WHO survey findings in 2002-2006 shows that MDR-TB, on average, is seen in 5.3% of all TB cases which are highest rates ever recorded of MDRTB.The results of drug susceptibility tests help to select a proper treatment regimen or modify treatment regimen for better management of patients and for surveillance and timely control of the spread of drug resistant TB in the community.
CONCLUSION We conclude that the MB/BacT system is an automated, rapid, and less labor-intensive mycobacterial culturing system which may be considered as a valuable alternative to the conventional culture.
REFERENCES
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home