Official Journals By StatPerson Publication
|
Table of Content Volume 12 Issue 3 - December 2019
Hitesh R Ahir*, Bhavesh P Gohil**
*Assistant Professor, **Tutor, Department of Microbiology, GMERS Medical College and Hospital, Gotri, Vadodara, Gujarat Email: bhaveshgohil08@gmail.com
Abstract Background: Hepatitis E infection, caused by the Hepatitis E virus (HEV), is a common cause of acute hepatitis in developing countries with poor sanitation and hygiene. The virus is classified into four genotypes (1–4) with one serotype. Genotypes 1 and 2 exclusively infect humans, whereas genotypes 3 and 4 also infect other animals, particularly pigs Aim: The aim of this study is to detect the prevalence of Hepatitis E viral infection in patients attending tertiary care teaching hospital. Material and Methods: The study was carried out from January 2018 to October 2018, to know the Hepatitis E viral infection prevalence from clinical samples in patients attending tertiary care teaching hospital. During this period total number of 421 serum samples received in the microbiology laboratory for Hepatitis E viral testing. All the samples received in microbiology laboratory were processed using standard microbiological guidelines for serological testing. Samples received in microbiology laboratory were processed by qualified and experience laboratory technician using HEV ELISA methods which can detect Anti HEV IgM antibody from patient’s serum. Results: During the study period total 421 samples were received in microbiology laboratory from patients attending tertiary care teaching hospital. Out of total no 421 samples received 69 samples were found to be positive by Anti HEV IgM ELISA methods. Out of them, 26 samples were positive from female patients and 43 samples were positive from male patients. Out of 69 total positive patients, 59 positive samples were during July to October 2018. Higher prevalence of HEV infection was noted in age group 11 to 40 years (n=51 positive samples). Conclusion: Seroprevalence of HEV viral infection is more during monsoon seasons (July to October months) in adults in age group 11 to 40 years as compare to others. This is because of poor sanitation and unhygienic conditions in developing countries like India. Key Words: Hepatitis E Virus infection, HEV ELISA, Anti HEV IG M antibody, Acute Viral Hepatitis(AVH).
INTRODUCTION Hepatitis E virus (HEV) which is the main aetiological agent of Enterically transmitted, non-A and non-C hepatitis, is highly endemic viral infection among the tropical and subtropical regions of Asia, Africa and South America.1 The Hepatitis E virus infection is mainly transmitted by faeco-oral routs mainly by contaminated water, but HEV may also be transmitted via food or blood transfusions, or vertically from mother to foetus 2. In most of the cases, the HEV infection is asymptomatic, the virus is spontaneously cleared from the host and only a few HEV cases may develop jaundice 3, 4, However, Sometime this HEV infection can be severe and accompanied by acute fulminant hepatitis. However, many widespread epidemics involving hundreds to thousands of people have been described in Asia, Africa, the Middle East and South America 7–9, with mortality rates varying from 0.2% to 4%. For some still unknown, but possibly immunological or hormonal reason 10, mortality is more frequent in pregnant women (10–20%), mainly those in the third trimester of pregnancy 11, 12. HEV genotypes appear to have a different geographic distribution and different clinical severity 1, 13: genotypes 1 or 2 are usually seen in developing countries and cause epidemic outbreaks. Genotype 1 is associated with vertical transmission 14. Genotype 3 is usually seen in developed countries; it does not cause outbreaks and the infection resolves without transmission to the infant 15, 16. Genotype 4 was found in sporadic cases of acute hepatitis E from China, Taiwan, Japan and Vietnam 13. In developed Western Countries, Seroprevalence of HEV is generally Viral hepatitis, caused by any of the five hepatotropic viruses, i.e., hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus (HEV), which contributed a major health problem worldwide. Among this viruses, HEV is now established as the major etiological agent of enterically transmitted non-A, non-B viral hepatitis. The first epidemic of hepatitis E infection in India was the epidemic of 1955–56 in New Delhi, affected a total of 29000 people; in which HEV was transmitted due to feacal contamination of drinking water. Although originally considered to be an epidemic of hepatitis A, retrospective testing of the stored sera from the affected patients suggested that a novel infectious agent was responsible17. Since the early 1990s, following the identification and sequencing of its etiological agent, the disease became known as hepatitis E and its agent as hepatitis E virus18. The letter ‘E’ stands for ‘enteric’, ‘epidemic’, or ‘endemic’, all of which are features that adequately describe the epidemiology of HEV. A high case fatality rate averaging around 20% in pregnant women, particularly in the third trimester, is a characteristic feature of HEV infection19,20. HEV is regarded as the major etiological agent of enterically transmitted non-A hepatitis in India21. HEV is responsible for both sporadic and epidemic outbreaks of acute hepatitis in developing countries, leading to a self-limiting disease. The global burden of HEV infection is more due to sporadically transmitted hepatitis E cases than to cases due to epidemic hepatitis E. Based on the 2010 global burden of diseases study, it has been estimated that as many as 20.1 million people were infected with HEV genotypes 1 and 2 in 2005, in nine regions. This represents 71% of the world’s population with 3.4 million symptomatic cases, 70,000 deaths, and 3000 stillbirths22. The death rate was higher among symptomatic pregnant women that among symptomatic non-pregnant women22. HEV has been classified as the type species of the new genus Hepevirus in the family Hepeviridae23. Study of the molecular biology of HEV was significantly advanced with the establishment of an efficient cell culture system24 and an effective vaccine25 but we still lack a reliable diagnostic procedure. However, anti-HEV antibody assays are widely available in European and Asian countries.
AIM The aim of this study is to detect the prevalence of Hepatitis E viral infection in patients attending tertiary care teaching hospital.
MATERIAL and METHODS The study was carried out from January 2018 to October 2018 to know the Hepatitis E viral infection prevalence from clinical samples in patients attending tertiary care teaching hospital. During this period total number of 421 serum samples received in the microbiology laboratory for Hepatitis E viral testing. All samples received in microbiology laboratory were processed using standard microbiological guidelines for serological testing. Samples received in microbiology laboratory were processed by qualified and experience laboratory technician using HEV ELISA methods which can detect Anti HEV IgM antibody from patients serum.
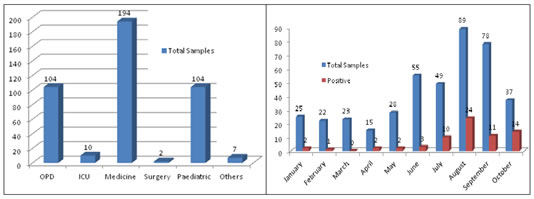
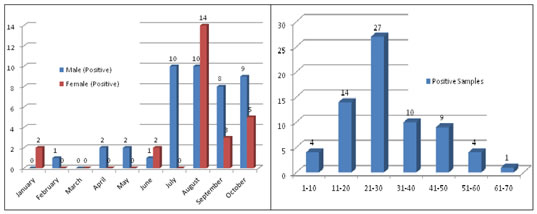
RESULT A total of 421 patients who presented with acute hepatitis were included and screened for hepatotropic viral markers. All of the patients had abnormal liver function tests suggestive of acute hepatitis, and were sporadic cases with no apparent links between patients. Although patients were identified in every month throughout the year, the incidence of HEV infection was highest from July to October 2018 (n=59 positive Samples 85.51%). There was no obvious variation in the incidence of HEV cases in other months. All of the 69 patients with HEV infection were icteric, but otherwise presented with different clinical signs and symptoms at the time of enrolment into the study. Overall prevalence of HEV in present study were 16.39%. Out of the 69 patients with HEV infection, 43 (62.32%) were male and 26 (37.68%) were female. The patients were aged 7–65 years. The age distributions of males and females were similar. The largest number of patients with HEV infection was seen in the age group 11–40 years (n=51 patients 73.91%). Table 1: Month wise Distribution of Anti HEV IgM Antibody Samples
Figure 1 Figure 2 Figure 3 Figure 4 Figure 1: Ward wise sample Distribution; Figure 2: Month wise Anti HEV IgM Antibody Samples (n=421), Positive=69; Figure 3: Month wise Male and Female Anti HEV IgM Antibody Positive samples; Figure.4: Age group wise distribution of Anti HEV IgM Antibody Positive samples DISCUSSION HEV is the most common cause of Acute Viral hepatitis(AVH) in developing countries, including India. HEV infection occurs in epidemics as well as sporadically, with periodic resurgences accounting for 30–70% of cases of acute sporadic hepatitis, regarded as a major cause of acute liver failure 26. Several studies of AVH from India have documented varying prevalences of HEV in sporadic cases 27, with results in accordance with those of the present study. The present study found that HEV primarily affects young adults between the ages of 11–40 years. Children younger than 7 years were excluded from the study, because anicteric hepatitis or subclinical infection is common in children younger than 7 years of age in cases of endemic hepatitis 28. An alternative explanation could be that HEV is maintained in the community as a sporadic infection; thus, HEV is acquired early in life, making infants and children immune to another attack 29. We also observed that HEV infection occurred throughout the year although seasonal variations in incidence were evident, with more patients with HEV infection from July to October than in any other month. This pattern is distinct from the epidemiological pattern in northern India reported by Aggarwal (2011)[30], suggesting that seasonal patterns can differ by geographical area.
CONCLUSION Seroprevalence of HEV viral infection is more during monsoon seasons (July to October months) in adults in age group 11 to 40 years as compare to others. This is because of poor sanitation and unhygienic conditions in developing countries like India.
REFERENCES
|
|
 Home
Home