|
Table of Content Volume 13 Issue 3- March 2020
Study of bacteriolgical profile of urinary tract infection in patients following instrumentation with special reference to ESBL Thipperudraswamy T1, Shilpa H S2*
1 Author, Associate Professor, Department of Microbiology, BLCMCRI, Bangalore, Karnataka, INDIA. 2Assisstant Professor, Department of Microbiology, Siddartha Medical College Tumkur. Email: trsganeshjai@gmail.com
Abstract Background: Urinary tract infection (UTI) has become the most common hospital acquired infection accounting for as many as 35% of nosocomial infections. Infection of one or more structures in the urinary system is called as UTI. Catheterisation and instrumentation are the major predisposing factors for UTI. Invasive urological procedures account for up to 10% of the nosocomial infections. β-Lactam antibiotics are commonly used to treat bacterial infections. ESBL are enzymes that mediate resistance to extended spectrum of antibiotics. e.g, third generation cephalosporins as well as Monobactams such as Aztreonam. Objective: To identify the causative organisms from urine sample of patients after instrumentation and there antimicrobial susceptibility pattern and to detect the ESBL production among the bacterial isolates. Materials and Methods: Samples were collected from 100 patients who underwent catheterisation and cystoscopy procedure. Samples were collected after two weeks in catheterised patients and 48hours of cystoscopy presenting with symptoms of urinary tract infections. Samples were processed using standard microbiological techniques. ESBL producers were detected among isolates according to CLSI guidelines. Results: Among 100 samples 38 isolates were isolated . Samples from catheterised patients showed maximum growth than cystoscopy patients . Age group between 40 to 50 years and males yielded maximum growth in both the procedures . E.coli was the predominate isolate, followed by Klebsiella spp, Citrobacter spp , Enterobacter spp ,Pseudomonas aeruginosa, Staphylocococcus aureus and CONS. Among38 isolates 34 isolates were Gram negative bacteria and 4 isolates were Gram positive cocci. Among 34 isolates 17 were ESBL producers. E.coli being predominate ESBL producer followed by Klebsiella spp ,Proteus spp, Citrobacter spp ,Enterobacter spp and Pseudomonas aeruginosa Conclusion: Statistical association of instrumentation of lower urinary tract with infection rate was not significant. Instrumentation is safe well tolerated diagnostic procedure, though there is minimal percentage of transient infection and unnecessary antibiotics need not be administered .These results suggest that prophylaxis is not needed for instrumentation in the absence of risk factors. Key Words: UTI, instrumentation, ESBL
INTRODUCTION Urinary tract infection(UTIs) is a term that is applied to variety of clinical conditions ranging from asymptomatic presence of bacteria in the urine to severe infection of the kidney with resultant sepsis1.Uriinary tract infections are among the most common bacterial infections both community and hospital setting.UTI has become the most common hospital acquired infection accounting for as many as 35% of nosocomial infections2. UTI is much more common in women than in men , due to anatomic and physiological reasons. UTI with increased risk include infants, pregnant women and the elderly, as well as those with indwelling catheters, diabetes, and underlying urological abnormalities.3 Intervention of urinary tract by various catheters, cystoscopes, guide wires and stents associated instrumentation is required for diagnostic, therapeutic purposes or both. Some of these procedures are employed as outpatient diagnostic procedure for variety of reasons in urology settings. Physicians should be familiar with the proposed instrumentation and they should make the patients aware of the procedure and its complications1 .Incidence of uro-genital tract infection in hospital environment is on the rise due to cross infection and lowered immune status of the patients3 .Up to 25% of hospitalised patients undergo urinary catheterisation, a similar proportions of patients have long term indwelling catheters. The overall incidence of nosocomial UTI among these patients is 35 to 10% ( average 5%) per day4 . The reported incidence of UTI after cystoscopy varies greatly between studies, with incidences between 21% and 0.85% being recorded5 .Common pathogens that have been implicated in UTIs are primarily Gram negative organisms with Escherichia coli having a more prevalence than other gram negative bacteria which include Klebsiella pneumoniae , Enterobacter spp, Proteus mirabilis , Pseudomonas aeruginosa and Citrobacter spp.6 β-Lactam antibiotics are commonly used to treat bacterial infections. The group of antibiotics in this category include Penicillins, Cephalosporins, Carbapenems and Monobactamas. Increased use of antibiotics, particularly the third generation of Cephalosporins ,has been associated with the emergence of β –lactamases mediated bacterial resistance, which subsequently led to the development of Extended spectrum of beta-Lactamases (ESBL)producing bacteria. ESBL are enzymes that mediate resistance to extended spectrum of antibiotics .e.g. third generation cephalosporins as well as Monobactams such as Aztreonam6.
MATERIALS AND METHODS Study period This prospective study was carried out in the Department of Microbiology, Vijayanagar Institute of Medical Science, Ballari over period of one year. This study was reviewed and approved by Institutional ethical committee, Vijayanagar Institute of Medical Science, Ballari. Patient’s undergoing instrumentation of lower urinary tract were taken under the study. Informed consent was obtained from study population. All patients satisfying the inclusion criteria were documented. SAMPLE COLLECTION Mid stream urine samples were collected from patients, catheterised for a period of two weeks7 and after 48 hours 8cystoscopy developed symptoms of urinary tract symptoms. Then samples sent to the microbiological laboratory in a wide mouthed universal container with a secured lid. Male patients were asked to retract the prepuce, cleanse the glans penis, with soap and water and then collect the sample from the middle urine flow. Female patients were instructed thoroughly to clean the ano-genital area from front to back, pass urine with labia separated and collect sample from middle portion of stream. Urine samples were collected from patients who has undergone instrumentation of lower urinary tract and presented with symptoms of urinary tract infection. Inclusion criteria
Exclusion criteria
STUDY SAMPLE PROCESSING Under strict aseptic precautions samples were collected from the patients and since urine is an excellent culture medium supporting rapid growth of many bacteria it was transported immediately to the laboratory in appropriate settings and samples processing was done within one hour. WET FILM EXAMINATION Urine sample was mixed carefully and about 0.05 ml of urine was placed in middle of the microscopic slide . At once a NO.1 cover slip of 22x22mm in dimension was placed over it , taking care to avoid air bubbles. The preparation was placed under high power objective [40x] of light microscope. The number of pus cells per high power field was recorded. Observation was also done for the presence of epithelial cells , red blood cells , parasites , yeasts(budding yeast cells and pseudohyphae) and bacteria. All these findings were recorded[9] . SEMIQUANTITATIVE CULTURE A calibrated loop that delivers 0.001 ml of urine was used to culture urine sample semi-quantitatively . A loopfull of urine was surface plated on CLED (cystine lactose electrolyte deficient agar ) . Urine sample was mixed thoroughly , the calibrated loop was inserted vertically in to the urine sample and the sample was inoculated on CLED media and streaked to obtain individual colonies9 .Urine samples will be collected from the patients having UTI symptoms after instrumentation under aseptic precautions . Sample processing will be acccording to standard microbiological culture methods (using MacConkey ,chacolate and CLED )to study their cultural characteristics. A single isolated colony will be considered for further studies, followed by identification using standard conventional ,morphological and biochemical test. Antimicrobial susceptibility will be tested by disc diffusion methods by Kirby-Bauer method. DETECTION OF ESBL: PHENOTYPIC METHODS:
A. Screening for ESBL producers:
B.Phenotypic confirmatory test:
The CLSI advocates use of cefotaxime (30μg) or ceftazidime discs (30μg) with and without clavulanate (10μg) for phenotypic confirmation of the presence of ESBLs. The CLSI recommends that the disk test be performed with confluent growth on MHA. A difference of ≥5mm between the zone diameters of either of the cephalosporin disc and their respective cephalosporin/clavulanate diskc is taken to be prolonged confirmation of ESBL production. II. Other methods for ESBL detection A. Modified double disc synergy test: Lawn culture of test strain on Muller Hinton agar was exposed to discs of Cefotaxime(30μg), Ceftazidime(30μg) and the disc of Amoxiclav(20μgamoxicillin/10μgclavulanic acid). The Cefotaxime and Ceftazidime disc were placed 16mm centre to centre from Amoxiclav disc. Plate was incubated at 370 c overnight. The test isolate was considered to produce ESBL , if the zone size around the Cefotaxime and Ceftazidime disc increased towards the Augmentin disc[10]. RESULTS A total of 100 midstream urine samples were processed from 100 patients each undergoing instrumentation (catheterisation, cystoscopy) presented with symptoms of lower urinary tract infection in hospitalised patients VIMS, BALLARI. From each patient clean catch midstream urine samples were collected under aseptic precautions. Patients having symptoms of lower urinary tract infection were subsequently excluded from the study.
TABLE 1: DISTRIBUTION OF PATIENTS WITH VARIOUS INSTRUMENT PROCEDURE
Among 100 urine samples collected 62 samples were collected from catheterised patients, among them 40 (64.5%) are males, 22(35.5%) are females. 38 samples are collected from post cystoscopy patients, among them 25(65.7%) were males and 13(34.5%) were females.
TABLE 2: AGE AND SEX WISE DISTRIBUTION OF PATIENTS
Among the 100 patients underwent instrumentation, of them were 65(65%) male patients and of them were 35(35%) female patients. Majority of the patients were from the 51-60 31(31%) age group followed by the age group 41-50 17(17%) in both the genders. Least number of patients were from<30 5(5%) age group and the 71-90 6(6%) genders.
TABLE 3: DIFFERENT PROCEDURES CONDUCTED ON PATIENTS SHOWING CULTURE POSITIVITY
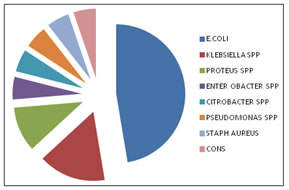
Above table shows among 100 patients 62 samples were collected from catheterised patient, out of 62 patients 25(40.3%) patients were culture positive .38 samples were collected from post cystoscopy patients, out of 38 patients 12(34.2%) patients were culture positive. GRAPH 1: DISTRIBUTION OF ISOLATES AMONG CULTURE POSITIVE PATIENTS
Majority of the isolates were E.coli18 (47.3%) followed by Klebsiella.spp6 (15.7%), Proteus spp4 (10.5%), Pseudomonas aeruginosa 2(5.2%) and Enterbacter spp2 (5.2%) Citrobacter spp2 (5.2%),CONS2(5.2%), Staph.aureus2(5.2%)
TABLE 4: COMPARISISON OF MODIFIED DDST AND PCT FOR ESBL DETECTION IN GRAM NEGATIVE ISOLATES
Among 34 isolates 23(67.6%) isolates showed resistance to 3rd generation Cephlosporins i.e screening method for ESBL detection 13(38.2%) isolates were positive modified double disk diffusion test with Amoxiclav . 17(50%) isolates were confirmed ESBL producer by phenotypic confirmatory test using Cefotaxime/Cefotaxime Clavulanic acid. DISCUSSION The instrumentation of lower urinary tract is done for diagnostic as well as therapeutic procedure. These procedure are commonly performed in VIMS BALLARI , of which urinary catheterisation and cystoscopy is commonly performed as day today procedure. Manipulation of lower urinary tract with instruments such as catheters ,cystoscopes, endoscopes , guide wires may result in various complication like pain ,discomfort, direct injury to tissues, haematuria ,urinary tract infection11 . In the hospitals the epidemiology has been investigated , 80% or more of the nosocomial UTIs are related to the use of urethral catheters . Another 5% to 10% occur after genitourinary manipulations .As a predisposing factor urological invasive procedure account for upto 10% of the hospital acquired infections12.In present study 100 urine samples are collected from patients who has undergone instrumentation procedure of lower urinary tract in VIMS BALLARI. This study include 62 post catheterised patients presented with symptoms of lower urinary tract symptoms after catheterisation > 2 weeks period.32 samples were collected from post cystoscopy patients after 48 hours of procedure , presented with symptoms of lower urinary tract infections. Mid stream urine samples were collected in a sterile container in department of Microbiology. Among 62 catherised patients 40 were male and 22 were female and in 38 post cystoscopy patients 25 were male and 13 were female .So the study includes 65 males and 35 females. The highest number was found in the age group 51-60 years about 28% followed by 31-40 years 24 %. Among 27 culture positive males >40years age group had higher culture positives (66.66%).than <40 years age groups (44.44%).Increased instrumentation and prostatic hypertrophy, decreased host defence account for bacteriuria in older age groups males13.In the present study the incidence of infection has been greater in males about 41.5% . The highest incidence of age group was seen in age group between 41-50 years(47%) . Pyuria is highly sensitive indicator of UTI but sometimes it as poor predictor of infection . It indicates only inflammation not always infection. In our study though all the samples showed pus cells only 38 samples were culture positive from 100 samples . So presence of pus cells may not be the reliable indicator of UTI in both sexes14,15. The highest incidence of infection with respect to instrumentation procedure was 27% by Mahim et al after catheterisation[4] ,38.75% by Wazat et aland 1625.7%with study done by Shilpa Gupta et al, 1758.5% by a study done by Ravichandraprakash et al13. In the present study the incidence of infection with catheterisation was 40% with catheterisation. The highest incidence of infection with respect to cystoscopy procedure was 34% by Steve et al 5,10.2% by Jimenez et al, 7.8% by Lugagne et al 19, 9% by Mark et al 207.5% by Burke et al[21]. In our study the incidence of infection with cystoscopy was 31.5% which correlates with other studies. The present study shows isolation of variety of organisms . In our study most predominate urinary pathogen isolated from both catheterisation and cystoscopy was E.coli (47.3%). This finding was similar to previous studies done by (53.3%) Mahim et al 5, (53%)Hale tauran etal 4, (50%) Kamil Cam et al3 and (80%) by Khanuengkiktong et al 24 .Where as Proteus mirabilis(65%) was predominate isolate in a study done by Harry et al 25. In our study other predominate pathogen were Klebsiella spp(15.7%) and Proteus spp(10.8%), Enterobacter spp(5.2%) ,Citrobacter spp (5.2%), Pseudomonas aeruginosa (5.2%), CONS(5.2%),Staph aureus(5.2%) was isolated from bothcatheterised and cystoscopy patients. In another study done by Wazit et al the isolatesafter catheterisation was E.coli (35.6%), Enterococcus(11.8%)40. E.coli (21.4%) and Candida spp (21%) followed by Enterococcus spp (10%) and Klebsiella pneumoniae (7.7%) ,Enterobacter (4%) was isolated in one more study incatheterised patients which correlates to findings in our study[20]. In another study Proteus mirabilis (88%), M.morgagani, P. staurti ,K. Pneumoniae ,P. rettgeri ,P.vulgaris were predominate isolates in catheterised patients 25.E.coli , Klebsiella pneumoniae, Proteus mirabilis , Providentia stuarti were isolated in one more study [26].E.coli , K pneumoniae , non Enterococcal group D Streptococcus were isolates in a cystoscopy patients study done by Hale tarun et al22. In a one more cystoscopy study done by A.Thompson the predominate isolates include E.coli followed by Staph .saprophyticus [27]. In our study 18E.coli isolates tested in our procedure study 39.9% were resistant to Gentamicin, 28% were resistant to Amikacin, 45% resistant to Cefotaxime and Ceftriaxone and 39% to Ceftazidime,39.9% to Ciprofloxacin .It showed low resistance to Nitrofurantoin (12.2%)and to Norfloxacin (3.9%) may be due to less frequent prescription of these drugs and these reports were similar to Biswas et al 28and Kauser et al 29. Low level of resistance to Amikacin was observed in our study compared to other studies. Low level of resistance of Norfloxacin was observed in our study similar to reports by Arjunan et al 30.In one more study E.coli was mostly sensitive to nitrofurantoin , amikacin and gentamicin and resistant Ampicillin , Ciprofloxacin and Cotrimoxazole31.Another study shows higher susceptibility percentage to Amikacin , Gentamycin and Levofloxacin .Higher resistance rate was noted for Amoxyclav , Ciprofloxacin, Cefotaxime ,Ceftazidime , Eratapenam. All isolates were found to be susceptible to imipenam and tigecycline32. Gupta V et al concluded that E.coli was 70 to 80% resistant to cotrimaxozole and aminopenicillin . However first generation Cephalosporins , Nitrofurantoin and Norflaxacin were effective but in cases where UTI was associated with other agents other than E.coli , Amikacin and third generation Cephalosporins were found to be effective33. Wazat H D et al concluded that there has been a change in the antimicrobial resistance, profile of various organisms .E.coli resistance to Co-amoxi clav and Ciprofloxacin has increased , and Enterococcal resistance to Ciprofloxacin has doubled and Nitrofurantoin remains unchanged over time 34. Mahim Koshariya et al concluded that antimicrobial resistance to commonly used antibiotics like ampicillin , trimethoprim and Gentamycin was high . Amakacin was found to have highest sensitivity (66.6%) followed by Nitrofurantoin (40.5%) Ceftazidime (33.3%) , Ofloxacin(26.2%), Ciprofloxacin(23.8%) and Cefaperazone +Sulbactam(26.2%) 4. The common pathogen isolated next to E. Coli was Klebsiella spp in our study and it is the same in most of the of the Indian studies . 50% of isolates exhibited resistance to Gentamycin i .e high level of resistance to Gentamycin compared to other drugs as in other studies .It showed low level of resistance to Amikacin(44%), Nitrofurantoin(17%)compared to other studies . The sensitivity pattern was similar to that of study done by P.Vasntha et al 35.In our study, of the 4 Proteus spp isolates all were sensitive to Amikacin whereas 33.3% of the isolates showed resistance to Gentamycin , Nitrofurantion, Norfloxacin. Resistance pattern to other drugs were Cefotaxime(66.6%), Ceftriaxone(33.3%), Ceftazidime(66.6%).The sensitivity pattern was similar to previous studies30. In our study of the 2 Citrobacter spp all were sensitive to Gentamycin,Nitrofurantoin, Norfloxacin and 50% of isolate showed resistant to Cefotaxime, Ceftriaxone, Ceftazidime .In one more study Citrobacter spp was responsible for about 20.68% of UTI were resistant to ampicillin in 100% of cases and susceptible to Norfloxacin in 83.33%37.In our study, of the 2 Enterobacter spp all were sensitive to Gentamycin, Nitrofurantoin, Norfloxacin and 50% of isolate showed resistant to Cefotaxime, Ceftriaxone, Ceftazidime .The sensitivity pattern was similar to previous studies36.one more in our study is Non Enterbactericeae group 2 Pseudomonas aeruginosa showed 100% resistance to Ciprofloxacin similar to the study by Bhargavi et al. . These 2 isolates sensitive to Gentamycin, 50% resistant to Norfloxacin , 50% resistant to Ceftazidime and 50% resistant to Ceftriaxone38. In our study, 2 isolate were CONS and 2 were Staph aureus which were sensitive to Nitrofurantoin ,resistant to Cotrimaxazole 50% resistant to Ampicillin and Erythromycin and all isolates sensitive to Methicillin and Norfloxacin .CONS 66.6% were sensitive to Nitrofurantoin and Ampicillin 33.3% sensitive to Ciprofloxacin and Ceftriaxone ,and 100 % sensitive to Vancomycin. In one more study , S. aureus was responsible for 1.72% of UTI and were resistant to Penicillin, Ampicillin, Nitrofurantoin and Tetracycline in 100% respectively39. A total of 34 gram negative bacteria, 23 (67.6%)were resistant to third generation cephalosporins. The isolates were tested for ESBL production by two methods. ESBL production was detected in isolates by modified DDST where as additional ESBL producers were detected by CLSI PCT .17(50%) isolates were ESBL producers by modified DDST and 19(55.8%)isolates were ESBL producers by phenotypic confirmation test. Various factors like precise placement of the discs, correct storage of the clavulanate containing disc and performance of appropriate controls tests are critical to the sensitivity of modified DDST 40 In comparison to this , phenotypic confirmation test is simple , cost effective and easy to perform therefore it can be used as routine test for ESBL detection. In our study maximum incidence of ESBL production was seen E.coli(58.8%) isolates, followed by Klebsiella spp ,(33.33%) then Proteus spp, (75%), Citrobacter spp(50%) Enterobacter(50%),Pseudomonas aeruginosa(50%). In a study done by Mahesh E et al the overall prevalence of ESBL was 66.78% and isolates ESBL positive was E.coli(66.77%), and Klebsiella spp (60.27%) which correlates with our study 39.In a study conducted by Aruna et almost common isolate was E.coli followed by Klebsiella pneumoniae, proteus, pseudomonas and study showed 72.05%of ESBL producers among isolates[40]. Iqbal M et al have reported ESBL productionin E. Coli ranging 25% in there study [41]. Highest prevalence rate of ESBL producingstrains have been reported in Klebsiella spp by Guptha et al33. Supriya et al conclude that 48% ESBL were produced in there study .E.coli, K.pneumoniae and Acinetobacter were ESBL producing species. Multi drug resistance was found to be significantly more in ESBL producing isolates(90.5%) than non ESBL producers(68.9%)[42]. In Uma Devi S [43] study,69% isolates were ESBL producer, consist of E.coli as (81.09%) major ESBL producer followed by K.pneumoniae(74.07%). Another study showed ,the rate of ESBL producer was 27.18% .E.coli(80%) is predominate isolate followed by Klebsiella spp(20%)[44].Nair T et al concluded that 56% of Uropathogenic was ESBL producers. High degree of antibiotic resistance to Gentamycin , Norfloxacin , Cotrimaxazole was seen among ESBL producers and non ESBL producers were sensitive to Imipenem(100%). ESBL producers were susceptible to Amikacin(84%), Nitrofurantoin(91%)respectively[45].
CONCLUSION
Instrumentation of lower urinary tract is one of the risk factor predisposes to complicated urinary tract infection. Catheter insertion and cystoscopy are widely used procedures for therapeutic and diagnostic purposes. In our study the incidence of UTI is slightly higher in males than females after instrumentation.The incidence of UTI is more in >40 years age group indicates age also plays significant role in incidence of UTI after instrumentation. Instrumentation is a safe and well tolerated diagnostic procedure, though minimum percentage of patients suffer from transient infection and unnecessary antibiotics need not to be administered..These results suggest that prophylaxis is not needed for instrumentation in the absence of risk factors.
REFERENCES
Policy for Articles with Open Access
|
|
 Home
Home