|
Table of Content Volume 13 Issue 3- March 2020
Bacterial and fungal isolates in corneal ulcers cases at tertiary care centre
Sunita R Bhandari1, Shubhra Sengupta2*
1Professor, 2Senior Associate Professor Department of Microbiology, SMBT Institute Of Medical Sciences And Research Centre, Dhamangoan, Nashik, Maharashtra, INDIA. Email: shubhrasengupta9@gmail.com
Abstract Background: Corneal blindness is the 4th major cause of blindness globally (5.1%) and ocular trauma and corneal ulcerations are significant causes. It is an ocular emergency, may rapidly progress to loss of vision. Role of microbiological assessment of aetiological factor and sensitivity to antibiotics is crucial. Aim: To assess risk factors and causative microbiological agents, to evaluate utility of Gram staining as a diagnostic test and to study the antimicrobial sensitivity pattern of bacterial isolates. Material and Methods: Descriptive cross-sectional study was carried out on 73 patients with the provisional diagnosis of corneal ulcers with infectious aetiology. Results: Male to female ratio was 1.8:1 with mean age of 35 years. Highest incidence was found in industrial labourers (38.5%) followed by housewives (23.25%). Staphylococcus epidermidis (34%), Staphylococcus aureus (22%) followed by Pseudomonas aeruginosa (14%) were commonest organisms isolated from growth. Sensitivity and Specificity of Gram staining with culture as a gold standard in present study were 64.44% and 96.43%. Out of 73 cases, 17.81% conjunctival swab showed growth and 61.64% corneal swab showed growth. Overall sensitivity ranged from 18% to 90% and resistance ranged from 10% to 82%. Bacterial isolates were sensitive to Sparfloxacin (90%) followed by Ciprofloxacin (70%) and resistant to Penicillin (82%) followed by Carbenicillin (71%). Conclusion: Microbiological work up in terms of Gram stain findings, results of culture and in-vitro studies of antibiotics sensitivities can be extremely useful in guiding the therapy of bacterial keratitis. Keywords: Microbial Keratitis, Infective Keratitis, Vision 2020, Preventable Blindness.
INTRODUCTION Corneal blindness is the 4th major cause of blindness globally (5.1%) after cataract, glaucoma and age-related macular degeneration (AMD). Ocular trauma and corneal ulcerations are significant causes of corneal blindness.1 Microbial keratitis should be considered a medical emergency. It is infection of the cornea caused due to broad spectrum of microbial agents.2 According to the World Health Organization (WHO), corneal blindness due to microbial keratitis is a ''silent epidemic'' happening unnoticed around the world.1,3 Corneal ulceration is a loss of corneal epithelium with clinical evidence of inflammation with or without hypopyon. It is an ocular emergency due to often rapid progression of this to the threat of blindness.4 Types of causative organism vary according to type of injury, climate and other sociodemographic parameters of person.5 Prompt and correct use of antibiotics is necessary to halt the diseases process and prevent complications. For this role of laboratory investigations is crucial which include culture of corneal scrapings and microscopy (gram staining) for identification of the causative microbial agent. Though culture is a gold standard but it takes longer time ranging from 48 hours to 14 days.6 Considering above facts, this study was planned to identify predisposing sociodemographic factors, to evaluate utility of Gram staining as a diagnostic test, to identify causative microbiological agents and to study the antimicrobial sensitivity pattern of bacterial isolates in this tertiary care hospital.
MATERIAL AND METHODS An observational descriptive cross-sectional study was carried out in a tertiary care hospital with a capacity of 1700 beds and super-speciality services in Cardiology, Nephrology, Urology, and Neurology. Institutional Ethics Committee (IEC) permission was taken before data collection. The study included 73 patients with the provisional diagnosis of corneal ulcers with infectious aetiology. Out of these, 22 patients were severe cases and required admission. Patients were excluded if they refused to participate, had viral ulcers, which were not secondarily, infected, Mooren's ulcer or if the patients were neonates. The patients were divided in to two groups i.e. definitive and probable, on the basis treatment taken prior coming to hospital. Definitive group comprised of those patients who could produce the antibiotics or knew the name and the probable group comprised of patients who had taken treatment but could not produce the name of antibiotic or at taken steroids or no treatment.7 Written informed consent was taken before data collection. Standard operating definitions and protocols were formulated and followed till end. The workup for each patient was carried out as per protocol which included a detail history especially with regard to risk factors and prior antibiotic therapy, a thorough clinical examination and ophthalmic assessment and laboratory procedures as per standard protocol. Details of the laboratory procedures:
Corneal scrapings were taken from the margins and centre of the ulcer by the ophthalmologist under slit lamp examination by sterile surgical blade No. 15 or by a small sterile needle. Multiple scrapings were done. The material is obtained from the leading edge of an active ulcer and also from deep down in the base of ulcer. For collection of conjunctival specimens the swabs were moistened in Brain Heart Infusion broth. The entire lower cul-de-sac is wiped.
Sample was first inoculated in the Brain Heart infusion Broth (BHI), Blood agar (BA) followed by Chocolate agar (CA) and Sabourauds Dextrose agar with Chloramphenicol (SDA). Lactophenol blue wet mounts, smears for gram stain, giemsa stain, kinon C cold carbafuchsin stain for Nocardia and Ziehl-Neelsen (20%) for mycobacteria were prepared from corneal scrapings and conjunctival swabs.[8]
For bacterial identification, Gram staining and for identification of fungi, lactophenol blue wet mounts and Giemsa staining were used.
The inoculation of corneal scrapings and conjunctival swabs was done on BHI (Brain Heart infusion broth), BA (Blood agar), CA (Chocolate agar), SDA (Sabouraud Dextrose agar).
If no growth was seen after 24 hrs. in blood agar, chocolate agar and brain heart infusion broth, the sample was reported as having no bacterial growth. If growth was presents its colony morphology was noted and a gram stained smear was prepared from the colony to study the morphology of the organism.
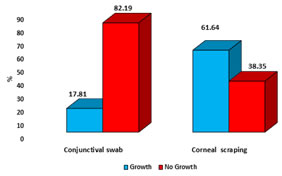
It was carried out according to standard Kirby Bauer disk diffusion method on Mueller - Hinton agar. The following standard strains were included as controls for antibiotic susceptibility testing: a) Gram positive - Staphylococcus aureus (ATCC 25923). b) Gram negative- Escherichia coli (ATCC 27853). c) Pseudomonas aeruginosa (ATCC 27853). The sensitivity results were interpreted as resistant, intermediate, sensitive, as per NCCLS standards (1997).9 Data was entered into Microsoft excel and analysed with SPSS. v.10. Descriptive statistics like frequency and proportion used at appropriate places. Tables and figures used to summarize the results. Sensitivity and specificity calculated to evaluate the diagnostic test. RESULTS Table no.1 depicts sociodemographic and injury profile of study subjects. Out of 73 cases of corneal ulcers, 64.38% were male and 35.62% were female with 1.8:1 as male to female ratio. Predominant age group was 20 to 50 years with average of 35 years. No case below the age of 10 years was reported. Proportion of corneal ulcer cases was more in each age group in males as compared to females. The highest incidence was found in industrial labourers (38.5%) followed by housewives (23.25%), students (19.17%), farmers (9.5%) and senior citizens (4.10%). Out of 73 cases of corneal ulcers, 66 (90.4%) had history of eye injury within last 3 months. Out of these 66 cases who had eye injury, 33 cases had injury due to foreign body and trauma, each. Vegetative foreign body injury responsible for 9.58% cases while surgical trauma was responsible for 4.1% cases. As shown in table no. 2, Staphylococcus epidermidis (34%), Staphylococcus aureus (22%) followed by Pseudomonas aeruginosa (14%) were the major organisms isolated in corneal ulcers. Other bacterial isolates were, Streptococcus pyogenes (6%), S. pneumonia (4%), S. viridians (4%), K. Pneumonia (4%) and E. Coli (4%). Out of total isolates from 73 cases, 45 (61.64%) isolates showed growth and 28 (38.35%) isolates showed no growth. Out of 45 cases, 5 (6.84%) showed mixed growth. In mixed growth isolates, S. aureus was associated with E. coli and S. pyogenes, S. epidermidis was associated with E. coli and K. pneumonia and S. pyogenes associated with P. aeruginosa. Among fungal isolates, Fusariaum, Candida species, Acremonium and Paecilomyces showed growth. Table no.3 highlights utility of Gram stained corneal smears against gold standard utility i.e. culture. Out of total 73 cases, 45 (61.64%) showed growth on culture while 41.09% cases were smear positive. Both were positive in 39.72% cases and both were negative in 36.98% cases. Only in one cases (1.36%), smear was positive but culture was negative. In 21.91% cases, culture were positive but smear results were negative. Sensitivity, Specificity, PPV and NPV of Gram staining with culture as a gold standard in present were 64.44%, 96.43%, 96.67% and 62.79%, respectively. Distribution of growth in conjunctival and corneal swab shown in fig. no. 1. Out of 73 cases, 17.81% conjunctival swab showed growth and 61.64% corneal swab showed growth. Out of the 13 conjunctival swabs; 4 (5.41%) cases were S. aureus, 9 (12.3%) cases were S. epidermidis. Out of the 4 (5.47%) cases of S. aureus cases, 3(4.1 %) were isolated in corneal scrapings. Out of the 9(12.3%) cases of S. epidermidis cases, 6(8.21%) were isolated in corneal scrapings. Comparison of sensitivity and resistance pattern of all bacterial isolates to different antibiotics shown in table no.4. As shown in table, overall sensitivity ranged from 18% to 90% and resistance ranged from 10% to 82%. Bacterial isolates were sensitive to Sparfloxacin (90%) followed by Ciprofloxacin (70%) and Netilmycin (60%). Bacterial isolates were resistant to Penicillin (82%) followed by Carbenicillin (71%) and Piperacillin (67%). Staphylococcus epidermis was most common organism isolated and highly sensitive to Sparfloxacin, Netilmycin and Ciprofloxacin. Staphylococcus aureus was highly sensitive to Netilmycin, Sparfloxacin and Piperacillin. Streptococcus species was highly sensitive to Erythromycin, Ciprofloxacin, Sparfloxacin and Cephalothin. Pseudomonas aeruginosa was sensitive to Ciprofloxacin, Tobramycin and Kanamycin while E. coli and K. pneumoniae sensitive to Netilmycin, Kanamycin and Sparfloxacin. Table 1: Sociodemographic and injury profile of patients with suppurative keratitis (n=73)
Table 2: Corneal Isolates from 73 cases of Corneal Ulcers / Suppurative Keratitis
Table 3: Evaluation of microscopy of Gram stained corneal smears compared with culture results
Figure 1: Isolates of corneal scraping and conjunctival swabs Table 4: Comparison of sensitivity and resistance pattern of all bacterial isolates to different antibiotics
DISCUSSION Study done by Sharma et al.10 reported, infectious keratitis in 61.58% males and 38. 42% females with Male: female ratio of 1.6:1. Commonest age group was 41-50 years (28.46%). In current study, male to female ratio was 1.8:1 with mean age of 35 years. Joshi et al.11 reported, 66.9% males and 33.08% female cases with most common age group between 30-60 years. Studies done by Seal et al.12 and Bashir et al.5 also reported similar findings. Males reported higher incidence as they are more exposed to the risk factor like trauma in outdoor activities. In present study, highest incidence was found in industrial labourers (38.5%) followed by housewives (23.25%). Ibanga et al.13 reported higher incidence in labours (29%) and students (19%) while Venkatesh et al.6 reported higher incidence in farm labourers (89.02%). Bashir et al.5 also reported highest incidence in farmers. Current study finding was slightly different from these study findings as study was conducted in urban area so most cases was of industrial labours. Study done by Seal et al.11 reported 44% cases due to ocular trauma while Jampla et al.14 reported 14% cases with trauma and 3% cases with post-operative trauma. In present study, trauma was cause for 45% cases and surgical trauma was cause for 4% cases. These findings were concurrent with the findings of Jampla et al.14. Sharma et al.10 reported concurrent findings with present study as vegetative cause of trauma reported in 33% cases and 45% has a history of trauma. In present study, Staphylococcus epidermidis (34%), Staphylococcus aureus (22%) followed by Pseudomonas aeruginosa (14%) were commonest organisms isolated from growth. Joshi et al.11 reported A. fumigatus (53%), staphylococcus (15%) and Streptococcus (13%) as commonest organisms. Sedhu et al.15 also reported Aspergillus and S. Pneumoniae as commonest isolates. Venkatlakshmi et al.4 reported concurrent findings with present study as they reported, S. Epidermidis (47%) and S. Aureus (21%) as commonest isolates. Jampla et al.14 reported P. Aeruginosa (24%) and S. Aureus (14%) as commonest bacterial isolates and Candida as a fungal isolates (44%). In current study, bacterial isolates were found in 46 cases and fungal isolates were in 4 cases. Jampla et al.14 reported fungal isolates in 12% cases. Study done by Seal et al.12 reported 10% mixed growth, 48% bacterial growth and 28% fungal growth. Ranjini et al.16 reported Fusarium and Aspergillus as commonest fungal isolates and S. aureus and P. Aeruginosa as commonest bacterial isolates. Sensitivity, Specificity, PPV and NPV of Gram staining with culture as a gold standard in present study were 64.44%, 96.43%, 96.67% and 62.79%. Study done by Jampla et al.14 reported, Gram stain’s sensitivity of 16.67% (95% CI: 4.84% to 37.40 %), specificity of 83.33% (95% CI: 36.10 % to 97.24 %), PPV (80%) and NPV (20%) in the case of bacterial infection. These findings were slightly different study findings. In present study, out of 13 conjunctival swabs; 4 (5.41%) cases were S. aureus, 9 (12.3%) cases were S. epidermidis. Out of these 9 isolates of S. epidermidis from conjunctival swabs, the same organism was isolated from corneal scrapping on culture in6 cases (8.21%) and similarly, out of 4 isolates of S Aureus from conjunctival swabs the same organisms was obtained from corneal scrapping in 3 cases (4.10%). Such high degree of agreement was not found between these two techniques for other bacteria especially gram-negative organisms. In current study, strains of S. Epidermidis, S. Aureus, P. Aeruginosa, S. Pyogenes, S. Pneumoniae, E. Coli, K. Pneumoniae showed maximum sensitivity to Sparfloxacin (88.2%), Netilmycin (90.9%), Ciprofloxacin (85.7%), Erythromycin (80%), Sparfloxacin (100%) and Netilmycin (100%), respectively. Bacterial isolates were resistant to Penicillin (82%) followed by Carbenicillin (71%) and Piperacillin (67%). Bacterial isolates were sensitive to Sparfloxacin (90%) followed by Ciprofloxacin (70%) and Netilmycin (60%). In a study done by Venkatlakshmi et al.[4], all Gram +ve bacteria were sensitive to moxifloxacin. They also reported, 89.33% sensitivity was seen in S. epidermidis isolates to Ciprofloxacin, Chloramphenicol, and Gatifloxacin. S. aureus strains were sensitive to Chloramphenicol (75%) and moxifloxacin (100%). Among the Gram -ve isolates, Pseudomonas aeruginosa exhibited good sensitivity to Ofloxacin (100%) and Chloramphenicol (100%). Study done by Seal et al.12, gram-negatives were sensitive to colistin and gram-positives to vancomycin and aminoglycosides. Gram-ve isolates were susceptible in highest percentage to moxifloxacin amikacin and meropenem (84.26% each) followed by gatifloxacin. Among gram-positive isolates, moxifloxacin showed sensitivity of 91.66% and gatifloxacin showed sensitivity of 94.43%. Moxifloxacin showed highest sensitivity against P.aeruginosa. All yeast isolates were sensitive to tested antifungal drugs. Overall, highest sensitivity showed by amikacin (92.06%) followed by gatifloxacin and gentamicin. Similar findings were reported by Ranjini et al.16 and Leck et al.17 in their studies.
CONCLUSION Microbiological work up in terms of Gram stain findings, results of culture and in-vitro studies of antibiotics sensitivities can be extremely useful in guiding the therapy of bacterial keratitis.
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home