|
Table of Content - Volume 20 Issue 1 - October 2021
Isolation and speciation of candida from various clinical samples by using conventional method and chrom agar in a tertiary care centre
Gunapravathy Istalingam I1, Dillirani Vedachalam2*, Sridhar Rathinam3
1Assistant Professor, Department of Microbiology, Government Thoracic Medicine Hospital/SMC Tambaram Sanitorium, Tambaram, Chennai, Tamil Nadu, INDIA. {2Professor & HOD, Department of Microbiology} {3Professor & HOD, Department of Chest & Respiratory Medicine} Government Stanley Medical College / GHTM, Chennai, INDIA. Email: dilliraniveda@gmail.com
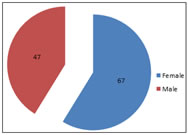
Abstract Background: Infections due to Candida especially Non-albicans Candida (NAC) species are increasingly being reported in recent years. Isolation and identification up to species level are of utmost importance in the early management of these infections. aim and objectives: To isolate and speciate Candida using germ tube test, chlamydospore formation test, sugar fermentation test, sugar assimilation test. To evaluate the performance of commercially available chromogenic Candida speciation media i.e HICHROM agar. Results: Among the total of 114 Candida spp., non-albicans candida species 64 (56%) were predominant compared to C.albicans 50 (44%). The age group above 60 years were commonly affected and females 67(59%) were more than male 47(41%). Among non-albicans candida species, C.tropicalis 48(42%) was predominant followed by C.glabrata 7(6%),C.krusei 6(5%) and C.parapsilosis 3(3%). The sensitivity and specificity of HICHROM agar was 100% when compared with conventional methods for isolation of Candida. Conclusion: The advantage of using CHROM agar is that it facilitates the isolation and identification of Candida to species level. The performance of CHROM agar exactly paralleled that of conventional methods. Use of this medium is rapid, technically simple, and cost-effective compared to the time-consuming technically demanding expensive conventional method. Key words: Candida albicans, Hicrome agar, Non albicans Candida.
INTRODUCTION Yeast-like fungi are ubiquitous. Yeast-like fungi particularly Candida species are the commonest cause of fungal infections. Candidiasis is a primary or secondary infection involving a member of the genus Candida, however, the disease is an infection caused by Candida albicans.1 Candida spp. is a member of the normal flora of the skin, mucous membranes, and gastrointestinal tract. They are endogenous opportunists which cause secondary infection in individuals with some underlying immunocompromised conditions. Candidiasis is a common fungal disease found in humans affecting mucosa, skin, nails, and internal organs of the body. Candida albicans is generally considered the major pathogen among the Candida species. An increase in the prevalence of non-albicans species has been noted during the last decades.2,3 There is growing evidence of the increasing use of azoles causing this epidemiological shift. Characterization to species level helps to identify those strains which might be intrinsically resistant to some of the antifungal agents.4,5 Speciation of Candida isolates is conventionally done by germ tube test, sugar assimilation, and sugar fermentation tests. Newer methods include CHROM agar, API systems, Vitek 2 ID system, and molecular methods.6,7 Germ tube test is a rapid method to differentiate C. albicans and C. dubliniens from other Candida spp. For further speciation chlamydospore formation test, sugar fermentation test, and sugar assimilation test can be done. But these tests are time-consuming and labor-intensive. Among the newer tests, CHROM agar is rapid and cost-effective as compared to other expensive systems like API systems, Vitek 2 ID system, and molecular methods.8
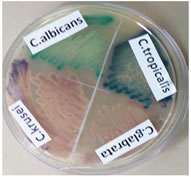
METHODS AND MATERIALS The study was carried out between November 2019 - 0ctober 2020 in the Department of Microbiology, Government Thoracic Medicine Hospital, Tambaram Sanitorium, Tambaram, Chennai after obtaining Ethical clearance. A total of 114 new consecutive Candida isolates from various clinical specimens like a high vaginal swab, midstream clean-catch urine, Catheterized urine, blood, sputum, pus, and skin scraping from patients with candidiasis were included in the study. Inclusion criteria: The study includes Candida isolates from various clinical samples sent routinely to the Microbiology laboratory. Exclusion criteria: Patients on any antifungal therapy 6 weeks prior to sample collection. These specimens were processed for the isolation of Candida spp. using standard Mycology methods. Gram staining was performed from the direct specimen and the specimens were inoculated on to Sabouraud’s dextrose agar slants, incubated at 37°C for 24 hrs. A germ tube test was done and the positives identified were either C. albicans or C. dubliniensis. C. albicans were further identified by growth at 45°C and Chlamydospore formation on cornmeal agar. All the 114 isolates were subjected to a Sugar fermentation test and Sugar assimilation test for final confirmation of species. Simultaneously the Candida spp. were inoculated on CHROM agar and incubated at 37°C for 24 hrs and the species were identified by type and color of the colonies on CHROM agar media as per the manufacturer’s instructions. (Table1)
Table 1: Colour of various Candia spp.on CHROM agar for identification
Figure 1: Gram positive budding yeast; Figure 2: Germ tube test positive Cells with pseudohyphae
Figure 3: Colour of Candida colonies on CHROM agar
RESULT A total of 114 Candida spp. Were isolated from a various clinical specimen. Among them non albicans candida species 64(56%), were predominant compared to C.albicans 50(44%).Non albicans Candida species included C.tropicalis 48(42%),C.glabreta 7(6%),C.krusei 6(5%) and C.parapsilosis (3%) Table 2: age group distribution
Table 3: Distribution of different species of Candida
Graph 1: Gender wise distribution of Candida
Table 4: Species distribution of Candida among various clinical sample
Table 5: Sensitivity and Specificity of CHROM agar for identification of various species of Candida
DISCUSSION Candida species colonize the mucosal surfaces of all humans soon after birth.3 The increasing incidence of iatrogenic Candida infections and the infections in immunocompromised and immunosuppressed individuals was due to the collective role of the fungal virulence factors and host susceptibility. In the immunocompetent host, several conditions predispose to fungal infections like prolonged antibacterial therapy, corticosteroid use, integumentary breach as in intravenous or intra-arterial catheters, surgical procedures, poor nutritional status, and metabolic derangements.3 The extensive use of antimycotic drugs for prolonged therapeutic courses led to a change in the relative prevalence of various species of Candida.1,7 Many studies in the past decade showed the isolation of various species of Candida and an increase in isolation of nonalbicans Candida in these situations and similar results were obtained in the present study. In our study, out of 114 Candida isolates, 64 (56 %) were non-albicans candida and 50(44%) were Candida albicans which was similar with the study by Rakeh Kumar Mukhia et al.13 reported 56% of non-albicans candida and 44% of Candida albicans. Jayalakshmi L et al.5 in her study documented 68.57% of non-albicans candida and 31.42% Candida albicans. However, Vaishali Wabale et al.24 and Shaheen MA et al.19 reported Candida albicans was higher in their study, 76% and 56% respectively. We observed that the frequent isolation of Candida species was in the age group above 60 years, 30% which was similar with the study of Rakeh Kumar Mukhia et al.13 who reported highest isolation of Candida in the age group above 60 years (34%) and R A Kashid et al.2 also reported Candida isolates was high in the age group above 60 years (24.48%). This could be due to lowered host defence at extremes of age. In the present study, most of the Candida isolates was found to be higher in female patients 67(59%) as compared to male 47(41%).This was similar with the study by Rakeh Kumar Mukhia et al.13 and Amar C S et al.1 who isolated candida species more from females 54(54%) and 62(60.2%) respectively than male patient 46(46%) and 41(39.8%) respectively. Our study differed from R A Kashid et al(12 who reported the isolation of Candida species was higher in Male 81(55.10%) as compared to females 66(44.8%).The reason for higher candida isolates in female is due to vaginal yeast infection, pregnancy and prolonged antibiotic therapy. In our study the incidence of non-albicans candida was 56% and C.albicans 44%.Various studies stated that the incidence of non-albicans candida ranges from 54-75%.20 A study by Vijaya et al.23 showed non-albicans candida (54.1%) to have a higher incidence than C.albicans(45.9%). A study by Manchanda et al.9 showed non-albicans candida (72.4%) to have a higher incidence than C.albicans (27.5%). Among the non-albicans species, Candida tropicalis (42%) was the predominant isolate followed by Candida glabrata (6%), Candida krusei (5%) and C. parapsilosis (3%). C. tropicalis is the most virulent of the non- albicans candida, this may be due to its ability to adhere to epithelial cells and its ability to secrete moderate levels of proteinases.12 In our study most of the Candida were isolated from Urine specimen (48%), followed by High vaginal swab (18%), sputum (17%), pus (10%) and skin scrapings(7%).A study by S. Mohan et al.11 says that most of the isolates were from Urine followed by respiratory specimen, pus, vaginal swab, CSOM, Blood and stool. However Jayalakshmi et al.5 isolated Candida more in sputum specimen (31%) followed by Urine specimen (28%). The conventional methods and the CHROM agar method were compared and were found to give similar results. This was similar to the findings of Sagar K et al.18 Nayaket al.14, who found that CHROM agar showed 100% specificity and 100% sensitivity when compared to SDA and conventional methods. The advantages of HICROM agar are easy to prepare, i.e. boiling, facilitate the rapid isolation and identification of yeast species. HICHROM agar facilitates identification between yeast spp. from specimens containing mixture of yeast spp. and do not affect the viability on subsequent subcultures. HICROM agar has the advantage of rapid identification of Candida spp, technically simple, rapid and cost-effective compared to technically demanding, time consuming and expensive conventional methods.
CONCLUSIONS Along with Candida albicans, non-albicans Candida spp like C. tropicalis, C. krusei, C. glabrata, and C. dubliniensis are increasingly being isolated from clinical samples. CHROM agar is a simple, rapid, and inexpensive method for the identification of such species. Characterization to species level helps to identify species that might be intrinsically resistant to commonly used antifungal agents. The accurate species identification of Candida is important for the treatment because not all species respond to the same treatment and also because of the increasing antifungal resistance. CHROMagar is a convenient and rapid method of identification of Candida species, especially in resource-limited poor settings.
References
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home