|
Table of Content - Volume 19 Issue 2- August 2021
Study of relation between neutrophil lymphocyte ratio, platelet lymphocyte ratio and gastric carcinoma outcome
Sandeep Jadhav1*, Hari Pada Mondol2
1Assistant Professor, Department of General Surgery, Lata Mangeshkar Hospital Digdoh, Nagpur, Maharashtra, INDIA. 2Professor, Department of General Surgery, Calcutta National Medical College and Hospital, 32, Gorachand Rd, Beniapukur, Kolkata, West Bengal 700014, INDIA.
Abstract Background: Gastric cancer represents a multifactorial histological change in which the interactions between environmental factors and genetics constitute an important part for its development and progression. Aim and objective: To elucidate the relation between neutrophil-lymphocyte ratio, platalet-lymphocyte ratio and gastric carcinoma outcome Methodology: Present study was prospective study carried out in patients with histopathologically diagnosed gastric carcinoma. CBC parameters including neutrophil, lymphocyte, monocyte, white blood cell (WBC) and platelet (Plt) count, hemoglobin (Hb) level were recorded, and the NLR and PLR calculated. NLR was defined as the ratio of absolute neutrophil count to absolute lymphocyte count; PLR was defined as the ratio of absolute platelet count to absolute lymphocyte count. Results and Discussion: The study revealed that higher NLR values are significantly associated with higher age of patient (p value 0.009), higher histological grade (p value 0.001), and higher stage of disease (p value 0.001). The study also revealed that higher PLR value are significantly associated with higher age of patient (p value 0.016), higher histological grade (p value 0.007) and higher stage of disease (p value 0.004).
INTRODUCTION Cancer disease, in general, is considered as one of the world’s major public health problems. Gastric cancer represents a malignancy that start in the stomach mucosa, being one of the most common sites of visceral malignant process. Gastric cancer is the fifth most common malignancy in the world, after cancers of the lung, breast, colorectum, and prostate1. However, compared to world statistics, India has quite a low incidence and prevalence of gastric cancers at 63,000 cases and 45,000 cases, respectively1. The incidence also varies widely among the different regions of India due to diverse sociocultural and eating habits. The initial diagnosis of gastric carcinoma is often delayed because up to 80 percent of patients are asymptomatic during the early stages of stomach cancer. 2 Weight loss, abdominal pain, nausea and vomiting, early satiety, and peptic ulcer symptoms may accompany late-stage gastric cancer. Signs may include a palpably enlarged stomach, a primary mass (rare), an enlarged liver, Virchow’s node (i.e., left supraclavicular), Sister Mary Joseph’s nodule (periumbilical), or Blumer’s shelf (metastatic tumour felt on rectal examination, with growth in the rectouterine/rectovesical space). UGI endoscopy is the diagnostic imaging procedure of choice in the work-up of gastric carcinoma. 3 It is a highly sensitive and specific diagnostic test, especially when combined with endoscopic biopsy. Although many biomarkers have been defined and studied in depth, excessive costs and technical factors often preclude their clinical use. Laboratory markers of systemic inflammation have been investigated as both prognostic and predictive biomarkers in several cancer populations. Assessment of the inflammatory response to the tumour may be easier and more-cost effective in clinical practice. Examples of these include CRP4, Glasgow Prognostic Score (GPS)5,6, neutrophil/lymphocyte ratio (NLR)7,8 and platelet/lymphocyte ratio (PLR)9,10 in predicting outcomes for patients after surgical resection but also in the patients with inoperable cancers. Although the causes of systemic inflammatory response (SIR) development in cancer patients are not fully understood, hypoxia secondary to tumour necrosis, alterations in neuroendocrine metabolism, synthesis of interleukin and production of acute phase proteins have been blamed11. Changes in white blood cell counts are reliable predictive markers for survival and therapeutical benefit in cancer patients12.This study was conducted to evaluate PLR and NLR correlations with the age and sex of patients, and outcome of gastric carcinoma. Aim and objective: To elucidate the relation between neutrophil-lymphocyte ratio, platalet-lymphocyte ratio and gastric carcinoma outcome
MATERIAL AND METHODS Present study was prospective study carried out in department of surgery at tertiary health care centre during period of June 2017 to May 2018. Study population was patients with histopathologically diagnosed gastric carcinoma Inclusion Criteria: 1. patients with histopathologically diagnosed gastric Carcinoma Exclusion criteria: 1. patients with secondary or priory malignancies 2. patients with hematological diseases 3.Patients with inflammatory diseases 4. patients undergone priory chemotherapy Study was approved by ethical committee of the institute. A valid written consent was taken from the patients after explaining study to them. Data was collected with pre tested questionnaire. Data included sociodemographic data like age, sex etc. Detailed clinical history was taken. A through clinical examination was done. For all patients disease stage, grade, a complete blood count (CBC) test were obtained before any treatments are applied. CBC parameters including neutrophil, lymphocyte, monocyte, white blood cell (WBC) and platelet (Plt) count, hemoglobin (Hb) level were recorded, and the NLR and PLR calculated. Patients follow up was kept for disease stage and grade which were obtained from CECT and UGI endoscopy biopsy report respectively and also for intra operative resectibility and post-operative resected specimen margins. NLR was defined as the ratio of absolute neutrophil count to absolute lymphocyte count; PLR was defined as the ratio of absolute platelet count to absolute lymphocyte count. In previous studies, a NLR cut-off value was used between 2.5-5.0 (Walsh et al., 2005; Yamanaka et al., 2007; Gomez et al., 2008; Aliustaoglu et al., 2010; Shimada et al., 2010; Unal et al., 2013) and a PLR cutoff value was used between 150-300 (Aliustaoglu et al., 2010; Unal et al., 2013; Templeton et al., 2014). We used the medians of distribution as the cut-off values. NLR was categorized into two groups (<2.75 and ≥2.75), and PLR was also categorized into two groups (<170 and ≥170). The data on categorical variables was shown as n (% of cases). The inter-group comparison of categorical variables was done using Chi-square test Or Fisher’s exact probability test for 2 x 2 contingency table when expected count was relatively small (<5)38. The entire data was entered and cleaned in MS Excel before its statistical analysis. All the results are shown in tabular form to visualize the statistically significant difference more clearly. In the entire study, the p-values less than 0.05 are considered to be statistically significant. All the hypotheses were formulated using two tailed alternatives against each null hypothesis (hypothesis of no difference)39,40. The entire data was statistically analyzed using Statistical Package for Social Sciences (SPSS ver 21.0, IBM Corporation, USA) for MS Windows.
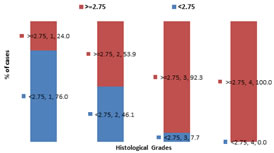
RESULTS In our study, majority of the patients were from the age below 60 years 34(62%) and patients above age of 60 years were 20(38%). The distribution of NLR levels differs significantly between group of cases with age less than 60 years and those with more than 60 years (P-value 0.009).About 75%(15) patients with age >=60yrs shows NLR value >=2.75 while only 38.2%(13) patients with age <60 yrs. The distribution of prevalence of high levels of NLR is significantly higher among the older cases (P-value<0.05). (table 1) In our study, male preponderance was observed. Out of total 54 patients, 30(55.5%) were male and 24(444.5%) were females. Study shows 60% (18) males and 41.6% (10) females with NLR value >=2.75 which is statistically insignificant(p value 0.180).The distribution of NLR levels did not differs significantly between group of male and female cases studied (P-value>0.05). (table 2) The distribution of NLR levels differs significantly across different histological grades in the study group (P-value 0.001).As per study high values of NLR can be seen in patients having high histological grade of stomach cancer (about 92.3% in grade 3 and 100% in grade 4) The distribution of prevalence of high levels of NLR is significantly higher among the cases with higher histological grades (P-value<0.05). (fig 1) The distribution of NLR levels differs significantly across different stages of cancer in the study group (P-value 0.001). The distribution of prevalence of high levels of NLR is significantly higher among the cases with higher stages of cancer (P-value<0.05). (table 3) The distribution of PLR levels differs significantly between group of cases with age less than 60 years and those with more than 60 years (P-value0.016). The distribution of prevalence of high levels of PLR is significantly higher among the older cases (P-value<0.05). (table 4) As per study 60% (18) males and 41.6% (10) females showing PLR value >=170 which is statistically insignificant(p value 0.180).The distribution of PLR levels did not differs significantly between group of male and female cases studied (P-value>0.05). (fig 2) The distribution of PLR levels differs significantly across different histological grades in the study group (P-value 0.007). The distribution of prevalence of high levels of PLR is significantly higher among the cases with higher histological grades (P-value<0.05). (table 5) The distribution of PLR levels differs significantly across different stages of cancer in the study group (P-value 0.004). The distribution of prevalence of high levels of PLR is significantly higher among the cases with higher stages of cancer (P-value<0.05). (table 6)
Table1: Associations of age with Neutrophil-to-lymphocyte ratio (NLR) in gastric carcinoma patients
Table 2: Associations of sex with Neutrophil-to-lymphocyte ratio (NLR) in gastric carcinoma patients
Figure 1: Associations of histological grade with Neutrophil-to-lymphocyte ratio (NLR) in gastric carcinoma patients
Table 3: Associations of stage of cancer with Neutrophil-to-lymphocyte ratio (NLR) in gastric carcinoma patients
Table 4: Associations of age with Platelet-to-lymphocyte ratio (PLR) in gastric carcinoma patients
Figure 2: Associations of age with Platelet-to-lymphocyte ratio (PLR) in gastric carcinoma patients
Table 5: Associations of age with Platelet-to-lymphocyte ratio (PLR) in gastric carcinoma patients
Table 6: Associations of age with Platelet-to-lymphocyte ratio (PLR) in gastric carcinoma patients
DISCUSSION In recent decades, our understanding of the inflammatory microenvironment of cancer has improved, and research has focused on the association between cancer and inflammation. Inflammation plays an important role in the development and progression of several cancers by suppressing or stimulating tumour cells. 13 Therefore, many inflammatory indicators, including NLR, platelet to lymphocyte ratio, CRP are diagnostic and prognostic biomarkers for various cancers.14 The study revealed that higher NLR values are significantly associated with higher age of patient (p value 0.009), higher histological grade (p value 0.001), and higher stage of disease (p value 0.001). The study also revealed that higher PLR value are significantly associated with higher age of patient (p value 0.016), higher histological grade (p value 0.007) and higher stage of disease (p value 0.004). Association of both NLR and PLR (p value 0.180 each) is insignificant with sex of patient. Chronic inflammation may be caused by Helicobacter pylori, and it is an important risk factor for stomach neoplasms. 15 However, the mechanisms involved in the association of elevated NLR and poor outcome for patients with gastric cancer remain unclear. A high NLR reflects a decrease in the number of lymphocytes and/or an elevated number of neutrophils. Neutrophils may play an important role in cancer development and progression by offering a suitable microenvironment for their growth. Circulating neutrophils may contain and secrete the majority of circulating vascular endothelial growth factor, interleukin-18, and matrix metalloproteinase, which are thought to be closely associated with tumourigenesis, development and metastasis. 16-18 Furthermore, the antitumor immune responses of activated T cells and natural killer cells may be inhibited by an elevated number of neutrophils surrounding tumour tissues. Therefore, a high level of circulating neutrophils may have a negative effect on patients with gastric cancer and lead to poor outcome. At the same time, lymphocytes play an important role in cellular adaptive immunity against cancer by attacking and clearing tumour cells at the outset of tumourigenesis.19 Although the NLRs were tested before treatment and status of patients was favourable, NLR still might be influenced by a number of confounding factors in peripheral blood. So the control of confounding factors in studies about the association between NLR and gastric cancer may be an important research point in the future. NLR can be considered as the balance between pro-tumour inflammatory status and anti-tumour immune status. Patients with elevated NLR have a relative lymphocytopenia and neutrophilic leukocytosis, which denotes that the balance is tipped in favour of pro-tumour inflammatory response and is associated with poor oncologic outcome. 7,8,20,21 Bambace N M demonstrated that platelets might stimulate tumour generation and promote metastasis by creating angiogenic factors, for example platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF). 22 In addition, a high platelet number would lead to relative lymphocytopenia, and the patient with cancer would have a hypoimmune response that is linked to lymphocyte-mediated antitumor activity at the cellular level. Platelets can trigger growth of the tumour by accelerating angiogenesis via cytokine vascular endothelial factor (VEGF) pathway. 23 When compared with healthy control group, considerable increases in VEGF-A levels in platelets of cancer patients have been demonstrated63. Significant association of NLR with tumour resectibility and post operative specimen margin positivity can be co related with overall survival of patients with gastric carcinoma on the other hand association of PLR with resectibility of tumour and specimen margin positivity is insignificant. In recent decades, a variety of predictors have been identified and applied for predicting GC outcomes. CEA, Her-2 are currently used in routine pathological assessment of GC. Ki-67, caspase-3 and p53 have also been reported associated with GC survival. 24 In addition, it is well known today that miRNAs have very important regulatory functions in cancer. Up to now, accumulating studies have investigated the diagnostic and prognostic values of miRNAs in GC. For example, Ueda T found that microRNAs are expressed differentially in gastric cancers and unique microRNAs are associated with progression and prognosis of GC. 25 However, the above-mentioned biomarkers should be examined in cancerous tissues. Thus it is impossible to monitor their levels continuously throughout disease progression. In contrast, NLR and PLR as indicators of inflammation can be easily assayed in plasma or serum, which may be widely applied in the clinic. The greatest limitation in our study was the diverse values of the NLR and PLR cutoff used in different studies previously. Our results are likely to be affected by the wide range of cutoff values for elevated NLR and PLR, which may affect the positive associations between NLR, PLR and GC prognosis. For example, cut-off scores of NLR were defined as 1.44, 2.5, 3.0,4.0 or 5.0 by analyzing the ROC curve, median value or based on previous studies, however, subgroup analyses stratified by cut-off values showed that the NLRs prognostic value was not affected substantially. In the future, studies with a larger sample size and more cancer types are needed for more reliable results.
CONCLUSION Hematological parameters including NLR and PLR were correlated with outcome in patients with gastric cancer.
REFERENCES
inoperable gastro-oesophageal cancer. Br J Cancer 2006, 94(5):637–641.
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home `
`