|
Table of Content - Volume 20 Issue 1 - October 2021
Study of prognostic value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients with gastric carcinoma
Sandeep Jadhav1, Hari Pada Mondol2*
1Assistant professor, Department of General Surgery, Lata Mangeshkar Hospital Digdoh Nagpur 440016, INDIA. 2 Professor, Department of General Surgery, Calcutta national medical College and Hospital, 32, Gorachand Rd, Beniapukur, Kolkata, West Bengal 700014, INDIA.
Abstract Background: For gastric cancer patients, the identification of sensitive markers that can predict prognosis and help select patients who may receive different treatments is needed. TNM staging is a good indicator for gastric cancer patients who undergo surgery41. Inflammation-based prognostic scores such as NLR and PLR could predict the prognosis of patients before they receive treatment. Aim and objective: To know prongostic value of nuetrophil-lymphocyte ratio and platelet-lymphocyte ratio in gastric carcinoma Methodology: Present study was prospective study carried out in patients with histopathologically diagnosed gastric carcinoma. All patients underwent Complete blood count. Haemoglobin, White blood cell count, neutrophil, lymphocyte, monocyte, and platelet count were noted. NLR and PLR calculated. NLR was defined as the ratio of absolute neutrophil count to absolute lymphocyte count; PLR was defined as the ratio of absolute platelet count to absolute lymphocyte count. Results and discussion: Higher NLR values are significantly associated with higher age of patient (p value 0.009), higher histological grade (p value 0.001), more depth of tumour invasion (p value 0.001), higher nodal status (p value 0.001), higher stage of disease (p value 0.001). Higher PLR value are significantly associated with higher age of patient (p value 0.016), higher histological grade (p value 0.007), more depth of invasion (p value 0.001), higher nodal status (p value 0.050) and higher stage of disease (p value 0.004)
INTRODUCTION India has quite a low incidence and prevalence of gastric cancers at 63,000 cases and 45,000 cases, respectively. 1 The consolidated Population Based Cancer Registry data of 2012–2014 shows highest age-adjusted rates at 40–50 in Northeastern states and 10.8 in Chennai2.Gastric cancer is one of the types of cancer with a higher mortality and diagnosis is usually made at a later stage3. As important prognostic factors, disease stage, histological type, margin of resection and in some studies age and gender of the patients have been reported4. Its higher mortality rates have led the investigators to search for other prognostic factors. Gastric cancer represents a multifactorial histological change in which the interactions between environmental factors and genetics constitute an important part for its development and progression. Thus, the incidence of gastric cancer is influenced by exogenous and endogenous factors. Identifying these factors result in understanding the molecular changes underlying the development of gastric cancer, but the etiology of gastric cancer is still obsucure at present.5 As with all types of cancer, the most important indicator of resectability and prognosis for gastric cancer is the clinicopathologic stage which can be assessed with the help of CT scan and other radiological modalities available like endoscopic ultrasound (EUS). Recently, changes in white blood cells in peripheral blood components as neutrophils, lymphocytes, monocytes and also neutrophil/lymphocyte ratio (NLR) estimates have been determined as simple, applicable, cost-effective and reliable prognostic indices.6-10 In addition to benign diseases as autoimmune disorders and infection, malignant diseases also induce chronic inflammation11,12. Therefore, the relationship between alterations in the microstructure of the tumour and prognosis has been investigated. Immune system cells as granulocytes and lymphocytes in the microenvironment of the tumour are important components of tumour stroma, which regulates carcinogenesis and development of metastases. Correlation between granulocytes and lymphocytes in the peripheral blood is closely related to these immune system cells.13 Platelet/lymphocyte ratio (PLR) and NLR have been demonstrated as important prognostic factors in some cancers including renal, gynecologic, pulmonary and colorectal cancers also prognostic role of PLR and NLR has been reported in GC. 12,14-18Our objectives in this study are to determine the correlation between PLR and NLR, which are calculated from routine whole blood parameters and prognosis of GC patients. Aim and objective: To know prongostic value of nuetrophil-lymphocyte ratio and platelet-lymphocyte ratio in gastric carcinoma
MATERIAL AND METHODS Present study was prospective study carried out in patients with histopathologically diagnosed gastric carcinoma. Study was carried out in department of surgery at tertiary health care centre during period of June 2017 to May 2018. Inclusion Criteria: 1. patients with histopathologically diagnosed gastric Carcinoma Exclusion criteria: 1. patients with secondary or priory malignancies 2. Patients with hematological diseases 3. Patients with inflammatory diseases 4. Patients undergone priory chemotherapy Study was approved by ethical committee of the institute. A valid written consent was taken from the patients after explaining study to them. Data was collected with pre tested questionnaire. Data included sociodemographic data like age, sex etc. Detailed clinical history was taken. A through clinical examination was done. All patients underwent Complete blood count. Haemoglobin, White blood cell count, neutrophil, lymphocyte, monocyte, and platelet count were noted. NLR and PLR calculated. NLR was defined as the ratio of absolute neutrophil count to absolute lymphocyte count; PLR was defined as the ratio of absolute platelet count to absolute lymphocyte count. NLR was categorized into two groups (<2.75 and ≥2.75), and PLR was also categorized into two groups (<170 and ≥170). Stage and grade of the disease was obtained by CECT and UGI endoscopy biopsy. Intra operative resectibility and post-operative resected specimen margins were recorded. Data was entered in excel sheet and analysed with SPSS version 20.0.
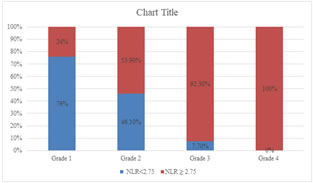
RESULTS In our study, majority of the patients were from the age below 60 years 34(62%) and patients above age of 60 years were 20(38%). The distribution of NLR levels differs significantly between group of cases with age less than 60 years and those with more than 60 years (P-value 0.009). About 75%(15) patients with age >=60yrs shows NLR value >=2.75 while only 38.2%(13) patients with age <60 yrs. Out of total 54 patients, 30(55.5%) were male and 24(44.45%) were females. Male to female ratio was 1.25:1. The distribution of NLR levels did not differs significantly with gender. (P-value>0.05). In our study, high values of NLR can be seen in patients having high histological grade of stomach cancer (about 92.3% in grade 3 and 100% in grade 4) The distribution of prevalence of high levels of NLR is significantly higher among the cases with higher histological grades (P-value<0.05). The distribution of prevalence of high levels of NLR is significantly higher among the cases with higher stages of cancer (P-value<0.05). The distribution of NLR levels differs significantly across different grades of depth of invasion in the study group (P-value 0.001). The distribution of prevalence of high levels of NLR is significantly higher among the cases with higher depth of invasion grades (P-value<0.05). The distribution of NLR levels differs significantly across different nodal grades in the study group (P-value 0.001). The distribution of prevalence of high levels of NLR is significantly higher among the cases with higher nodal grades (P-value<0.05). The distribution of NLR levels differs significantly across different stages of cancer in the study group (P-value 0.001). The distribution of prevalence of high levels of NLR is significantly higher among the cases with higher stages of cancer (P-value<0.05). The distribution of NLR levels differs significantly between group of cases with positive and negative specimen margin in the study group (P-value 0.001). The distribution of prevalence of high levels of NLR is significantly higher among group of cases with positive specimen margin (P-value<0.05). The distribution of prevalence of high levels of PLR is significantly higher among the older cases (P-value<0.05). As per study 60% (18) males and 41.6% (10) females showing PLR value ≥ 170 which is statistically insignificant(p value 0.180). The distribution of PLR levels differs significantly across different histological grades in the study group (P-value 0.007). The distribution of prevalence of high levels of PLR is significantly higher among the cases with higher histological grades (P-value<0.05). The distribution of PLR levels differs significantly across different grades of depth of invasion in the study group (P-value 0.001). The distribution of prevalence of high levels of PLR is significantly higher among the cases with higher depth of invasion grades (P-value<0.05).
Figure 1: Distribution of levels of NLR according to histological grade
Table 1: Distribution of levels of NLR according to depth of invasion
Table 2: Distribution of levels of NLR according to Nodal status
Table 3: Distribution of levels of NLR according to stage
Table 4: Distribution of levels of PLR according to Depth of invasion
Table 5: Distribution of levels of PLR according to Nodal status
Table 6: Distribution of levels of PLR according to stage
DISCUSSION NLR, in particular, is a prognostic indicator for several other solid cancers such as urinary 19 and colorectal cancer 20,21. In my study, I demonstrated that the prognosis of patients with high NLRs was worse than that for patients with a normal NLR amongst gastric cancers patients. Furthermore, I found that high NLRs are associated with late-stage gastric cancer, more tumour invasion, higher nodal status, unresectibility and post operative margin positivity. There are several explanations for the correlation between poorer prognosis and elevated NLR in gastric cancer. Patients who have lymphocyte infiltration surrounding their tumours may have a better prognosis than those with less or no infiltration 22. In addition, lymphocytes may be suppressed by large numbers of neutrophils when two cells are co cultured 23. Our results indicate that an elevated NLR denotes a pretreatment inflammatory condition that is correlated with poor prognosis for patients with gastric cancer. Platelet count is an additional index of systemic inflammation elicited by the tumour. This analysis demonstrated that a high PLR is linked to a higher risk of lymph node metastasis, and the high PLR also increased the serosal invasion (T3 +T4) risk and the advanced stage (III +IV) risk in patients with gastric cancer. PLR is associated with prognosis of many types of cancer including colorectal, pulmonary and hepatocellular cancers. However, specific mechanism of this correlation has not been fully understood. 24 Nieswandt B proved that platelets are capable of protecting tumour cells from cytolysis and can promote metastasis. Surface shielding by integrin αIIbβ3 (glycoprotein IIb/IIIa) bridging seems to be the main mechanism of this protection 25, and platelets can also secrete inflammatory proteins such as IL-6, TNF-α, et al., which have also been linked to tumour cell metastases 26,27. In addition, by the release of secretory factors that promote growth factors, chemokines, proangiogenic regulatory proteins, proteolytic enzymes and microparticles within the microenvironment, activated platelets promote tumour cell growth and invasion28 In my study I found that high PLR is associated with higher tumour invasion, higher nodal involvement, later stage of disease but no significant correlation found between PLR and resectibility of tumour and post operative margin positivity. Platelet aggregation and degranulation along with the consequent release of platelet-derived proangiogenic mediators within the microvasculature of the tumour also could be an important determinant of tumour growth 29. No clear picture is available on the significance of tumour-platelet interactions. A number of proinflammatory mediators are known to stimulate megakaryocyte proliferation 30. Smith et al. have defined preoperative PLR as an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma.31 Recently, it was noted that inflammation and the interaction between various inflammatory cells and the extra-cellular matrix plays a crucial role in the tumour micro-environment of tumourigenesis, progression and metastasis 32-36. The peripheral blood count can partly reflect the inflammatory response and is routinely conducted with no need for additional effort in patients. In addition, it is convenient and inexpensive 37. There are two potential interacting mechanisms between inflammation and cancer. First, by generation of reactive oxygen species and pro inflammatory cytokines, inflammation may slowly initiate oncogenesis 33,35. Recently, Qian BZ and Pollard JW demonstrated that at the early stage of the neoplastic progression, inflammation definitely promoted benign neoplasms to cancers 36. On the other hand, cancer could generate inflammation and that inflammation could then promote low grade malignancies to transition to states of heightened malignancy by genetic evolution 34,38. Based on these studies, research has attempted to identify the prognostic role of various inflammation-based factors including the PLR, NLR and the platelet count in cancer patients. There were several limitations in this study. Firstly, this analysis was constrained to only 54 patients and only those who attended our institution, so centre bias could not be excluded. Secondly, there are no randomized controlled trials (RCTs), however, our conclusion is stable with low heterogeneity.
CONCLUSION Hematological parameters like NLR and PLR were correlated with prognosis in patients with gastric cancer.
REFERENCES
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home